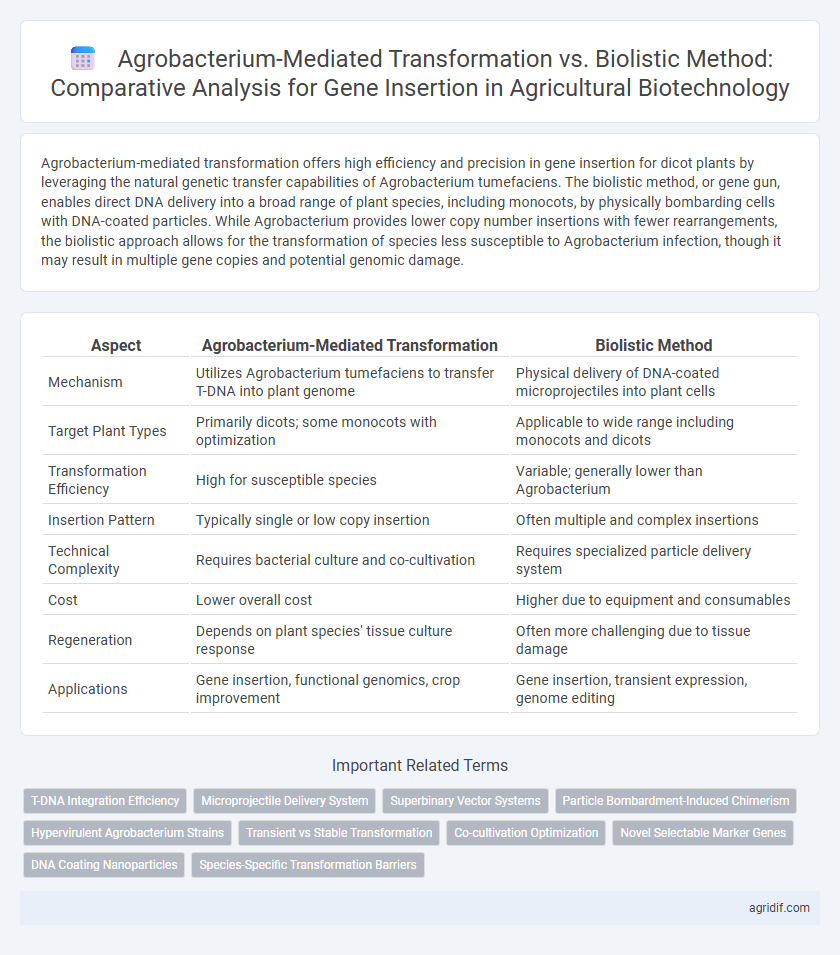
Agrobacterium-mediated transformation offers high efficiency and precision in gene insertion for dicot plants by leveraging the natural genetic transfer capabilities of Agrobacterium tumefaciens. The biolistic method, or gene gun, enables direct DNA delivery into a broad range of plant species, including monocots, by physically bombarding cells with DNA-coated particles. While Agrobacterium provides lower copy number insertions with fewer rearrangements, the biolistic approach allows for the transformation of species less susceptible to Agrobacterium infection, though it may result in multiple gene copies and potential genomic damage.
Table of Comparison
| Aspect | Agrobacterium-Mediated Transformation | Biolistic Method |
|---|---|---|
| Mechanism | Utilizes Agrobacterium tumefaciens to transfer T-DNA into plant genome | Physical delivery of DNA-coated microprojectiles into plant cells |
| Target Plant Types | Primarily dicots; some monocots with optimization | Applicable to wide range including monocots and dicots |
| Transformation Efficiency | High for susceptible species | Variable; generally lower than Agrobacterium |
| Insertion Pattern | Typically single or low copy insertion | Often multiple and complex insertions |
| Technical Complexity | Requires bacterial culture and co-cultivation | Requires specialized particle delivery system |
| Cost | Lower overall cost | Higher due to equipment and consumables |
| Regeneration | Depends on plant species' tissue culture response | Often more challenging due to tissue damage |
| Applications | Gene insertion, functional genomics, crop improvement | Gene insertion, transient expression, genome editing |
Overview of Gene Insertion Techniques in Agriculture
Agrobacterium-mediated transformation harnesses the natural ability of Agrobacterium tumefaciens to transfer T-DNA into plant genomes, offering precise gene insertion with minimal copy number and stable expression in dicotyledonous crops. The biolistic method, or gene gun, propels DNA-coated microprojectiles into plant cells, enabling direct gene delivery across a wider range of species, including monocots and recalcitrant crops where Agrobacterium is less effective. Both techniques remain fundamental in crop genetic engineering, influencing trait introduction such as pest resistance, herbicide tolerance, and abiotic stress resilience.
Introduction to Agrobacterium-Mediated Transformation
Agrobacterium-mediated transformation utilizes the natural ability of Agrobacterium tumefaciens to transfer T-DNA into plant genomes, enabling precise gene insertion with minimal copy number and stable expression. This method is widely preferred for dicotyledonous plants due to its high efficiency and reduced genomic rearrangements compared to biolistic methods. Key advantages include lower transgene silencing and fewer mutations, making it a cornerstone technique in crop genetic engineering.
Fundamentals of the Biolistic (Gene Gun) Method
The biolistic method, or gene gun technique, employs high-velocity microprojectiles coated with DNA to physically penetrate plant cell walls and insert genetic material directly into the target cells. Unlike Agrobacterium-mediated transformation which relies on bacterial infection mechanisms, the biolistic approach allows for the transformation of a wider range of plant species, including monocots and recalcitrant crops. This physical delivery system facilitates transient and stable gene expression, making it pivotal in functional genomics and the development of genetically modified plants with desirable agronomic traits.
Mechanisms of DNA Delivery: Agrobacterium vs Biolistics
Agrobacterium-mediated transformation exploits the natural ability of Agrobacterium tumefaciens to transfer a specific DNA segment, the T-DNA, into the plant genome through a wound-induced infection process, ensuring targeted and stable gene integration. In contrast, the biolistic method employs high-velocity microprojectiles coated with DNA to physically penetrate plant cell walls, facilitating random DNA delivery into the cell nucleus without biological vectors. The precision of Agrobacterium's T-DNA insertion contrasts with the more variable and often multiple copy insertions characteristic of the biolistic approach, influencing transgene expression stability.
Efficiency and Success Rates in Crop Species
Agrobacterium-mediated transformation exhibits higher efficiency and success rates in dicotyledonous crops such as tomatoes and soybeans due to its natural ability to transfer DNA, yielding stable gene integration with fewer copy numbers. The biolistic method, while broadly applicable to monocots like maize and wheat, often results in lower transformation efficiency and more complex integration patterns, causing variable gene expression. Agrobacterium's targeted insertion minimizes gene silencing compared to the biolistic approach, which frequently generates multiple transgene copies leading to rearrangements and reduced transgene stability.
Limitations and Challenges of Each Method
Agrobacterium-mediated transformation faces limitations such as host specificity, primarily affecting dicotyledonous plants, and complex regulatory concerns due to possible T-DNA integration variability. The biolistic method overcomes host range restrictions but presents challenges including physical damage to plant tissues and random, multiple gene insertions that can lead to unstable expression. Both techniques require optimization to balance transformation efficiency, gene expression stability, and minimal genomic disruption in crop genetic engineering.
Plant Host Range and Compatibility
Agrobacterium-mediated transformation offers a broad plant host range, especially effective in dicotyledonous species due to its natural ability to transfer T-DNA into plant genomes, ensuring high compatibility and stable gene integration. In contrast, the biolistic method, or gene gun technique, provides versatility across monocots and dicots by physically delivering DNA, bypassing host specificity but sometimes causing random gene insertion and multiple copy integration. The choice between these methods hinges on crop species, desired gene expression stability, and transformation efficiency related to plant tissue compatibility.
Applications in Modern Crop Improvement
Agrobacterium-mediated transformation is widely utilized for precise gene insertion in dicotyledonous crops, enhancing traits such as disease resistance and stress tolerance through the efficient transfer of T-DNA into plant genomes. The biolistic method, or gene gun, enables direct DNA delivery into diverse crop species, including monocots like maize and wheat, facilitating genetic modifications in species less susceptible to Agrobacterium infection. Both techniques play crucial roles in modern crop improvement by enabling the development of genetically engineered plants with improved yield, pest resistance, and environmental adaptability.
Biosafety and Regulatory Considerations
Agrobacterium-mediated transformation offers higher biosafety due to its natural gene transfer mechanism, minimizing unintended genetic rearrangements, which often aligns with regulatory preferences for precision and reduced off-target effects. The biolistic method, while versatile across plant species, poses greater biosafety concerns because of random DNA integration and potential tissue damage, complicating regulatory approvals. Regulatory frameworks emphasize thorough risk assessment of gene insertion techniques, favoring approaches with established safety records and predictable transgene expression patterns.
Future Prospects: Emerging Trends and Innovations
Agrobacterium-mediated transformation continues to advance through CRISPR-Cas9 integration, enhancing precise gene editing in diverse crops. Biolistic methods are evolving with nanotechnology, improving DNA delivery efficiency and minimizing tissue damage. Emerging trends emphasize combining both techniques for multiplex genome engineering, promising breakthroughs in crop yield and stress resistance.
Related Important Terms
T-DNA Integration Efficiency
Agrobacterium-mediated transformation exhibits higher T-DNA integration efficiency due to its natural mechanism for transferring DNA into plant genomes, resulting in more stable and precise gene insertion compared to the biolistic method. The biolistic method often causes multiple copy insertions and random integration, which can lead to transgene rearrangement and reduced expression stability.
Microprojectile Delivery System
The Microprojectile Delivery System, also known as the biolistic method, uses high-velocity microcarriers to physically penetrate plant cell walls for gene insertion, offering direct DNA delivery without the host specificity constraints of Agrobacterium-mediated transformation. This technique is particularly advantageous for transforming monocots and recalcitrant species, enabling stable integration of foreign genes across diverse crop genomes.
Superbinary Vector Systems
Superbinary vector systems enhance Agrobacterium-mediated transformation efficiency by increasing T-DNA transfer capacity, outperforming the biolistic method in stable gene integration and reduced copy number insertion. These vectors leverage additional virulence genes to improve transformation rates in recalcitrant plant species, providing a more precise and cost-effective approach compared to the physical DNA delivery in biolistics.
Particle Bombardment-Induced Chimerism
Particle bombardment-induced chimerism in biolistic gene insertion arises from the heterogeneous integration of DNA into plant cells, often leading to mosaic tissues with both transformed and non-transformed cells. In contrast, Agrobacterium-mediated transformation typically results in more uniform gene integration, reducing chimerism and enhancing stable transgene expression in agricultural biotechnology applications.
Hypervirulent Agrobacterium Strains
Hypervirulent Agrobacterium strains enhance gene insertion efficiency in Agrobacterium-mediated transformation by promoting higher T-DNA transfer rates and stable integration into plant genomes, outperforming the biolistic method in transformation fidelity and lower tissue damage. These strains are particularly advantageous for crops recalcitrant to genetic modification, enabling precise transgene delivery with reduced off-target effects compared to particle bombardment techniques.
Transient vs Stable Transformation
Agrobacterium-mediated transformation excels in stable gene insertion by integrating DNA into the plant genome, enabling heritable trait expression, whereas the biolistic method often results in transient gene expression due to random DNA delivery and lower efficiency in stable integration. The choice between these techniques hinges on the desired outcome: Agrobacterium is preferred for producing genetically stable transgenic plants, while biolistics suits transient assays and applications requiring rapid gene expression without genome integration.
Co-cultivation Optimization
Co-cultivation optimization in Agrobacterium-mediated transformation enhances T-DNA transfer efficiency by fine-tuning factors such as bacterial density, infection duration, and acetosyringone concentration to improve gene insertion success rates in plant tissues. Compared to the biolistic method, this approach requires precise control of co-cultivation conditions to maximize stable gene integration while minimizing tissue damage and transformation-associated stress.
Novel Selectable Marker Genes
Agrobacterium-mediated transformation utilizes natural bacterial mechanisms to insert novel selectable marker genes efficiently into plant genomes, often resulting in lower copy number insertions and more stable gene expression compared to the biolistic method. The biolistic method, while capable of delivering multiple gene constructs including advanced selectable markers, may cause complex integration patterns and higher genetic rearrangements, impacting the precision of gene insertion in agricultural biotechnology.
DNA Coating Nanoparticles
Agrobacterium-mediated transformation leverages natural T-DNA transfer mechanisms, offering high gene integration efficiency with minimal DNA damage, whereas the biolistic method employs high-velocity particle bombardment, often using gold or tungsten nanoparticles coated with DNA to physically insert genes into plant cells. DNA coating on nanoparticles enhances protection and stability of genetic material during delivery, critically influencing transformation success rates and expression consistency in both techniques.
Species-Specific Transformation Barriers
Agrobacterium-mediated transformation exhibits high efficiency in dicotyledonous plants due to species-specific recognition and infection mechanisms, whereas monocots often present barriers such as low susceptibility and defense responses. The biolistic method bypasses these barriers by physically delivering DNA into plant cells, making it a versatile alternative for species with recalcitrant Agrobacterium-host interactions.
Agrobacterium-Mediated Transformation vs Biolistic Method for Gene Insertion Infographic
 agridif.com
agridif.com