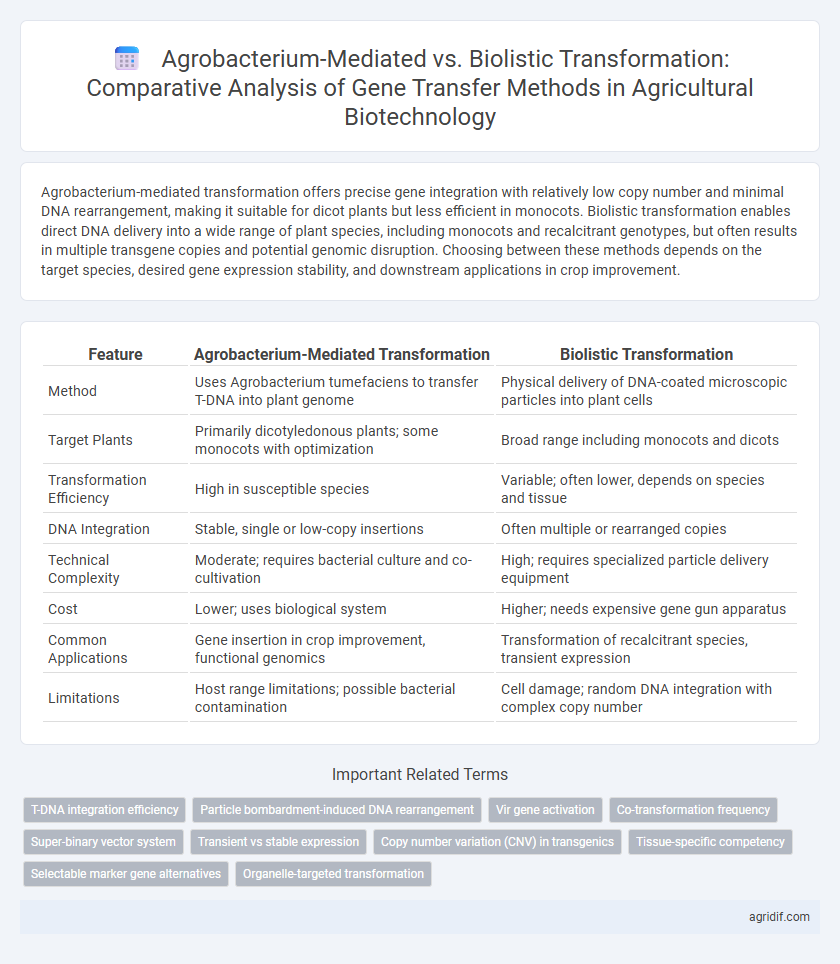
Agrobacterium-mediated transformation offers precise gene integration with relatively low copy number and minimal DNA rearrangement, making it suitable for dicot plants but less efficient in monocots. Biolistic transformation enables direct DNA delivery into a wide range of plant species, including monocots and recalcitrant genotypes, but often results in multiple transgene copies and potential genomic disruption. Choosing between these methods depends on the target species, desired gene expression stability, and downstream applications in crop improvement.
Table of Comparison
| Feature | Agrobacterium-Mediated Transformation | Biolistic Transformation |
|---|---|---|
| Method | Uses Agrobacterium tumefaciens to transfer T-DNA into plant genome | Physical delivery of DNA-coated microscopic particles into plant cells |
| Target Plants | Primarily dicotyledonous plants; some monocots with optimization | Broad range including monocots and dicots |
| Transformation Efficiency | High in susceptible species | Variable; often lower, depends on species and tissue |
| DNA Integration | Stable, single or low-copy insertions | Often multiple or rearranged copies |
| Technical Complexity | Moderate; requires bacterial culture and co-cultivation | High; requires specialized particle delivery equipment |
| Cost | Lower; uses biological system | Higher; needs expensive gene gun apparatus |
| Common Applications | Gene insertion in crop improvement, functional genomics | Transformation of recalcitrant species, transient expression |
| Limitations | Host range limitations; possible bacterial contamination | Cell damage; random DNA integration with complex copy number |
Overview of Gene Transfer Methods in Agriculture
Agrobacterium-mediated transformation utilizes the natural ability of Agrobacterium tumefaciens to transfer DNA into plant genomes, offering high transformation efficiency and stable gene integration predominantly in dicotyledonous plants. Biolistic transformation, or particle bombardment, employs high-velocity microprojectiles to deliver DNA directly into plant cells, enabling gene transfer across a wider range of plant species, including monocots and recalcitrant crops. Both methods are fundamental in developing genetically modified crops for traits such as pest resistance, herbicide tolerance, and improved nutritional content, with selection depending on plant species, transformation efficiency, and desired genetic outcomes.
Introduction to Agrobacterium-Mediated Transformation
Agrobacterium-mediated transformation utilizes the natural ability of Agrobacterium tumefaciens to transfer a segment of its DNA, known as T-DNA, into the plant genome, enabling stable gene integration. This method is favored for its high efficiency in dicotyledonous plants and precise gene insertion with minimal copy number variation. Compared to biolistic transformation, Agrobacterium-mediated transformation reduces the occurrence of transgene rearrangement and is widely employed for genetic engineering in crops like tobacco, tomato, and soybean.
Principles of Biolistic (Gene Gun) Transformation
Biolistic transformation utilizes high-velocity microprojectiles, typically gold or tungsten particles, coated with DNA to physically penetrate plant cell walls and deliver genetic material directly into the genome. This technique bypasses the host range limitations of Agrobacterium-mediated transformation, enabling gene transfer in monocots and recalcitrant species. Key parameters influencing transformation efficiency include particle size, bombardment pressure, target tissue type, and DNA loading density on microcarriers.
Mechanisms of DNA Delivery: Agrobacterium vs Biolistics
Agrobacterium-mediated transformation utilizes the natural ability of Agrobacterium tumefaciens to transfer T-DNA into plant genomes via its type IV secretion system, ensuring precise and stable gene integration. Biolistic transformation, or particle bombardment, physically propels DNA-coated microprojectiles into plant cells, enabling direct DNA delivery but often resulting in multiple and random integration sites. The distinct molecular mechanisms influence transformation efficiency, gene copy number, and expression stability in genetically engineered crops.
Transformation Efficiency: Comparative Analysis
Agrobacterium-mediated transformation generally exhibits higher transformation efficiency in dicotyledonous plants due to its natural ability to transfer T-DNA into plant genomes, achieving transformation rates up to 80% in some crops like tobacco and tomato. In contrast, biolistic transformation, or particle bombardment, is more versatile across monocots and recalcitrant species but often shows lower efficiency, ranging between 5-30%. The choice between these methods depends on target species, with Agrobacterium preferred for stable gene integration and biolistics favored for direct DNA delivery in cereals such as maize and wheat.
Range of Host Species and Applicability
Agrobacterium-mediated transformation primarily targets dicotyledonous plants due to its natural infection mechanism, making it highly effective for crops like tomatoes, potatoes, and tobacco. Biolistic transformation, also known as the gene gun method, offers broader applicability across both monocots and dicots, including cereals like maize and rice that are typically resistant to Agrobacterium infection. The choice between these methods depends on the plant species and desired transformation efficiency, with biolistics enabling genetic modification in a wider range of host species.
Integration Patterns and Transgene Expression
Agrobacterium-mediated transformation typically results in lower copy number transgene integration with more precise insertion patterns, leading to more stable and predictable transgene expression. In contrast, biolistic transformation often generates complex, multi-copy insertions with rearrangements that can cause variable or silenced transgene expression. The integration pattern's stability directly influences long-term transgene expression levels and phenotypic consistency in genetically modified crops.
Advantages and Limitations of Each Method
Agrobacterium-mediated transformation offers high transformation efficiency and stable gene integration primarily in dicot plants, benefiting from its natural ability to transfer T-DNA into the host genome. However, its limitations include host range restrictions and reduced effectiveness in monocot species. Biolistic transformation, compatible with a broader spectrum of plant species including monocots, allows direct DNA delivery to cells but often results in multiple transgene copies and potential tissue damage, impacting stable gene expression and plant regeneration.
Current Applications in Crop Improvement
Agrobacterium-mediated transformation is widely used for introducing desired genes into dicotyledonous crops due to its high efficiency and stable gene integration, playing a crucial role in developing pest-resistant and herbicide-tolerant varieties. Biolistic transformation, or gene gun technology, enables gene transfer in monocots and recalcitrant species where Agrobacterium is less effective, facilitating improvements in cereal crops like maize and rice. Both methods contribute significantly to crop improvement by enhancing yield, stress tolerance, and nutritional quality in commercially important plants.
Future Trends and Innovations in Plant Genetic Engineering
Agrobacterium-mediated transformation offers precise gene integration with reduced copy number and is preferred for dicotyledonous crops, while biolistic transformation enables gene transfer in monocots and recalcitrant species. Future trends emphasize combining CRISPR/Cas systems with both methods to enhance targeted genome editing and increase transformation efficiency. Innovations include developing novel Agrobacterium strains and optimized particle delivery systems to expand host range and reduce off-target effects in plant genetic engineering.
Related Important Terms
T-DNA integration efficiency
Agrobacterium-mediated transformation demonstrates higher T-DNA integration efficiency due to its natural ability to transfer and integrate genetic material into plant genomes with minimal DNA rearrangement. Biolistic transformation often results in lower integration efficiency and more complex, fragmented T-DNA insertions, which can affect stable gene expression.
Particle bombardment-induced DNA rearrangement
Particle bombardment-induced DNA rearrangement often results in complex insertions and multiple copy integrations, causing unpredictable gene expression in biolistic transformation. Agrobacterium-mediated transformation typically produces fewer rearrangements with more stable, single-copy gene insertions, enhancing transgenic plant reliability.
Vir gene activation
Agrobacterium-mediated transformation exploits the natural mechanism of Vir gene activation by acetosyringone compounds to facilitate T-DNA transfer into plant genomes, ensuring high efficiency and stable integration. Biolistic transformation bypasses biological signaling pathways, delivering DNA via particle bombardment without Vir gene involvement, often resulting in multiple or fragmented insertions and lower transformation precision.
Co-transformation frequency
Agrobacterium-mediated transformation typically exhibits higher co-transformation frequency due to its natural mechanism of transferring multiple T-DNA segments into plant genomes, enhancing the stability and efficiency of gene integration. In contrast, biolistic transformation often results in lower co-transformation frequency with fragmented or multiple copies of genes being randomly inserted, which can lead to gene silencing or unpredictable expression patterns in plants.
Super-binary vector system
The Agrobacterium-mediated transformation using a super-binary vector system enhances gene transfer efficiency by carrying multiple virulence genes, facilitating higher DNA integration rates compared to traditional binary vectors. Unlike biolistic transformation, which physically bombards plant cells with DNA-coated particles, the super-binary system promotes stable transgene expression and lower copy number insertions, reducing genome disruption in transformed crops.
Transient vs stable expression
Agrobacterium-mediated transformation enables efficient stable gene integration by transferring T-DNA into plant genomes, ensuring heritable expression, whereas biolistic transformation often results in transient expression due to random DNA delivery and variable integration. Stable expression is favored in Agrobacterium methods for durable trait inheritance, while biolistics is useful for rapid transient assays to assess gene function without genomic integration.
Copy number variation (CNV) in transgenics
Agrobacterium-mediated transformation typically results in lower copy number variation (CNV) with more stable integration of transgenes, whereas biolistic transformation often produces high CNV and complex integration patterns due to multiple DNA insertions. Lower CNV in Agrobacterium-mediated methods correlates with reduced gene silencing and enhanced transgene expression stability in agricultural biotechnology.
Tissue-specific competency
Agrobacterium-mediated transformation demonstrates high tissue-specific competency by efficiently targeting dicotyledonous plant cells, particularly in leaf and stem tissues, due to its natural infection mechanism. In contrast, biolistic transformation offers broader applicability across diverse plant tissues, including monocots and recalcitrant cells, but often results in random DNA integration and variable tissue-specific expression.
Selectable marker gene alternatives
Agrobacterium-mediated transformation typically utilizes antibiotic resistance genes such as nptII or hpt as selectable markers, while biolistic transformation often requires additional optimization due to variable DNA integration and expression patterns. Alternatives like herbicide resistance genes (bar, ALS) and visual markers (GUS, GFP) provide effective selection in both methods, reducing reliance on traditional antibiotic resistance markers and addressing biosafety concerns in agricultural biotechnology.
Organelle-targeted transformation
Agrobacterium-mediated transformation enables precise integration of foreign genes primarily into the nuclear genome but faces challenges in targeting organelles like chloroplasts or mitochondria. Biolistic transformation, utilizing high-velocity microprojectiles, is more effective for organelle-targeted gene transfer, facilitating direct delivery of DNA into chloroplasts and mitochondria, thereby enhancing the potential for stable organelle genome modification in plants.
Agrobacterium-mediated transformation vs biolistic transformation for gene transfer Infographic
 agridif.com
agridif.com