Chelated micronutrients provide enhanced bioavailability and stability compared to inorganic salts, ensuring efficient nutrient uptake in plants. Their complexed form prevents rapid precipitation and soil fixation, leading to better absorption and reduced nutrient loss. This results in improved plant growth, higher yields, and greater resilience against environmental stresses.
Table of Comparison
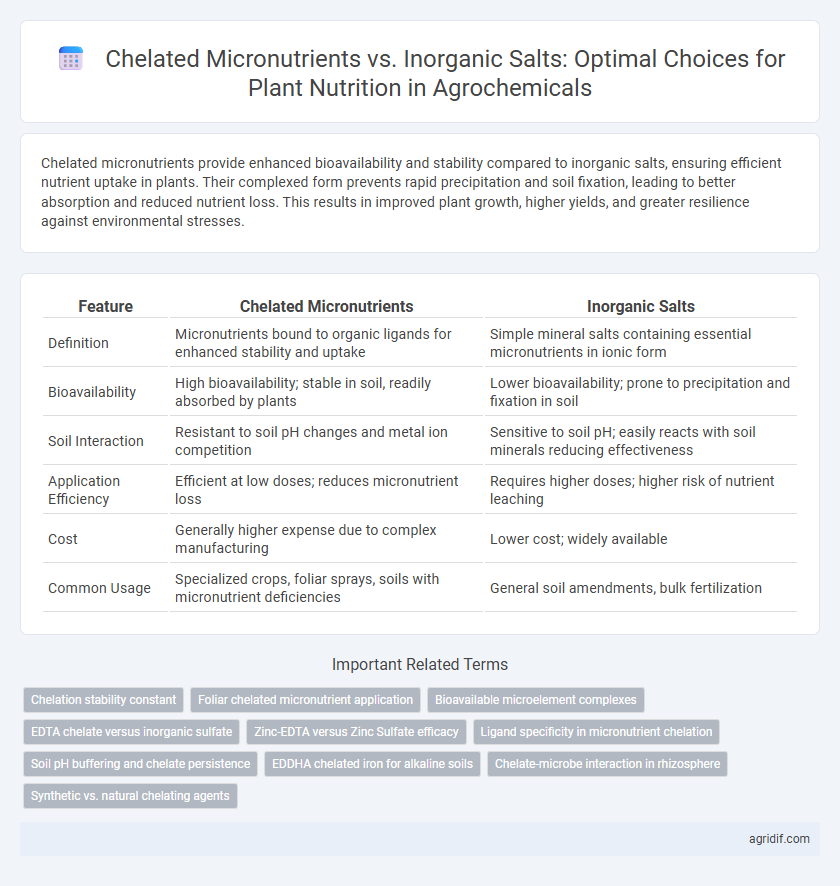
| Feature | Chelated Micronutrients | Inorganic Salts |
|---|---|---|
| Definition | Micronutrients bound to organic ligands for enhanced stability and uptake | Simple mineral salts containing essential micronutrients in ionic form |
| Bioavailability | High bioavailability; stable in soil, readily absorbed by plants | Lower bioavailability; prone to precipitation and fixation in soil |
| Soil Interaction | Resistant to soil pH changes and metal ion competition | Sensitive to soil pH; easily reacts with soil minerals reducing effectiveness |
| Application Efficiency | Efficient at low doses; reduces micronutrient loss | Requires higher doses; higher risk of nutrient leaching |
| Cost | Generally higher expense due to complex manufacturing | Lower cost; widely available |
| Common Usage | Specialized crops, foliar sprays, soils with micronutrient deficiencies | General soil amendments, bulk fertilization |
Introduction to Chelated Micronutrients and Inorganic Salts
Chelated micronutrients, such as EDTA and DTPA complexes, enhance nutrient availability by preventing precipitation and improving uptake efficiency in plants compared to inorganic salts like sulfates and chlorides. Inorganic salts often suffer from limited solubility and increased susceptibility to soil pH variations, which reduce their bioavailability. The superior stability and mobility of chelated micronutrients facilitate more consistent micronutrient delivery, promoting optimal plant growth and yield.
Chemical Structure and Composition Differences
Chelated micronutrients consist of metal ions bound to organic ligands forming stable ring-like structures that enhance nutrient availability and uptake in plants. Inorganic salts contain metal ions paired with simple anions, lacking the complex molecular framework of chelates, often resulting in lower bioavailability due to precipitation or fixation in soil. The chemical stability and solubility of chelated micronutrients make them more effective in delivering essential trace elements compared to inorganic salts in diverse soil conditions.
Mechanisms of Nutrient Uptake in Plants
Chelated micronutrients enhance nutrient uptake by maintaining metal ions in soluble, bioavailable forms, preventing precipitation and fixation in the soil, unlike inorganic salts which often form insoluble compounds. Plant roots absorb chelated nutrients more efficiently through active transport mechanisms facilitated by stable chelate complexes that protect micronutrients from antagonistic interactions. This results in improved translocation within the plant's vascular system, leading to higher micronutrient use efficiency compared to conventional inorganic salt fertilizers.
Bioavailability and Efficiency in Soil Environments
Chelated micronutrients exhibit higher bioavailability compared to inorganic salts due to their stable molecular structure, which prevents precipitation and fixation in diverse soil environments. This enhanced solubility enables efficient nutrient uptake by plant roots, especially in alkaline or calcareous soils where inorganic salts often become insoluble. The precise delivery mechanism of chelates supports sustained nutrient release, improving overall plant nutrition and growth efficiency.
Stability and Solubility Under Various Conditions
Chelated micronutrients exhibit superior stability and solubility compared to inorganic salts under diverse soil pH and moisture conditions, ensuring consistent nutrient availability for plants. The chelation process prevents micronutrient precipitation and immobilization, optimizing uptake efficiency in acidic, alkaline, or calcareous soils. Inorganic salts, prone to rapid dissolution and leaching, often lead to nutrient loss and reduced bioavailability, particularly in fluctuating environmental conditions.
Impact on Crop Yield and Plant Health
Chelated micronutrients enhance crop yield and plant health by improving nutrient availability and absorption efficiency compared to inorganic salts, which often suffer from precipitation and reduced bioavailability in soil. The stable organic complex in chelates prevents micronutrient fixation, ensuring consistent uptake and preventing deficiencies that limit plant growth. Research shows crops treated with chelated micronutrients exhibit stronger resistance to stress and higher productivity, making them essential for optimized agricultural nutrition management.
Compatibility with Other Agrochemicals
Chelated micronutrients exhibit superior compatibility with a wide range of agrochemicals compared to inorganic salts, minimizing precipitation and antagonistic reactions in tank mixes. Their stable molecular structure enhances nutrient availability and reduces the risk of nutrient lockout when combined with pesticides or fertilizers. Using chelated forms improves overall efficacy and ensures consistent nutrient uptake in integrated crop management systems.
Environmental Implications and Soil Health
Chelated micronutrients enhance nutrient uptake by plants more efficiently than inorganic salts, reducing the risk of nutrient leaching and soil contamination. Inorganic salts often contribute to soil acidification and disrupt microbial activity, negatively impacting soil health and long-term fertility. Selecting chelated micronutrients supports sustainable agriculture by promoting balanced nutrient availability and preserving soil ecosystem integrity.
Cost-Effectiveness and Economic Considerations
Chelated micronutrients offer higher bioavailability and reduced nutrient loss compared to inorganic salts, often resulting in improved crop yield and quality, which can justify their higher upfront cost. Inorganic salts are generally cheaper but exhibit lower efficiency due to precipitation and fixation in soil, necessitating increased application rates and potentially raising overall input expenses. Economic considerations favor chelated forms in high-value crops and precision agriculture, where their targeted delivery enhances cost-effectiveness by minimizing nutrient waste.
Practical Applications and Recommendations for Farmers
Chelated micronutrients enhance nutrient uptake efficiency in plants compared to inorganic salts by maintaining nutrient availability across varying soil pH levels, reducing leaching and fixation issues. Practical application involves foliar sprays or soil drenching with chelated forms like EDTA or DTPA to improve micronutrient assimilation, particularly in alkaline or calcareous soils where inorganic salts may precipitate. Farmers should prioritize chelated micronutrients for targeted deficiencies, ensuring precise dosage and timing to optimize growth and yield outcomes in diverse agro-ecological zones.
Related Important Terms
Chelation stability constant
Chelated micronutrients exhibit higher stability constants than inorganic salts, enhancing nutrient availability and uptake efficiency in plants by preventing premature precipitation and soil fixation. Stability constants for common chelates like EDTA range from 10^16 to 10^25, significantly outperforming typical inorganic salt complexes with stability constants below 10^5.
Foliar chelated micronutrient application
Foliar chelated micronutrient application enhances nutrient uptake efficiency by delivering bioavailable metals directly to leaf tissues, minimizing soil fixation issues common with inorganic salts. Chelated forms improve micronutrient stability and mobility, ensuring consistent absorption critical for optimal plant growth and stress resilience.
Bioavailable microelement complexes
Chelated micronutrients provide higher bioavailability compared to inorganic salts due to their stable molecular structure that prevents nutrient precipitation and enhances absorption efficiency in plant roots. These bioavailable microelement complexes deliver essential elements like iron, zinc, and manganese more effectively, promoting improved plant growth and resistance to nutrient deficiencies.
EDTA chelate versus inorganic sulfate
EDTA chelated micronutrients offer superior bioavailability and stability compared to inorganic sulfate salts, enabling plants to efficiently uptake essential elements like iron and zinc under various soil pH conditions. Unlike inorganic sulfates, EDTA chelates minimize nutrient precipitation and leaching, ensuring sustained micronutrient supply for optimized plant growth and yield in agrochemical applications.
Zinc-EDTA versus Zinc Sulfate efficacy
Chelated micronutrients like Zinc-EDTA offer higher bioavailability and sustained release compared to inorganic salts such as Zinc Sulfate, enhancing zinc uptake efficiency in plants. Zinc-EDTA minimizes soil fixation and pH sensitivity, resulting in improved nutrient mobility and better correction of zinc deficiency in diverse soil conditions.
Ligand specificity in micronutrient chelation
Ligand specificity in micronutrient chelation enhances nutrient stability and bioavailability, as chelated micronutrients form stronger, more selective complexes compared to inorganic salts, preventing precipitation and facilitating efficient plant uptake. This selective binding improves micronutrient transport within plant tissues, optimizing nutrient delivery and minimizing soil interactions that reduce nutrient effectiveness.
Soil pH buffering and chelate persistence
Chelated micronutrients exhibit enhanced soil pH buffering capacity and longer chelate persistence compared to inorganic salts, ensuring sustained nutrient availability in diverse soil conditions. The stable chelation prevents micronutrient precipitation and immobilization, optimizing uptake efficiency in acidic to alkaline soils.
EDDHA chelated iron for alkaline soils
EDDHA chelated iron significantly improves iron availability in alkaline soils by preventing precipitation and enhancing absorption compared to inorganic iron salts, which often become insoluble and ineffective. Chelated micronutrients like EDDHA ensure sustained nutrient release and higher bioavailability, optimizing plant growth and preventing chlorosis in high pH environments.
Chelate-microbe interaction in rhizosphere
Chelated micronutrients enhance bioavailability and stability in the rhizosphere by forming complexes that prevent nutrient precipitation and facilitate microbial uptake mechanisms. These interactions promote beneficial microbial activity, improving nutrient solubilization and uptake efficiency compared to inorganic salts, which often exhibit lower stability and reduced microbial synergy.
Synthetic vs. natural chelating agents
Synthetic chelating agents, such as EDTA and DTPA, enhance micronutrient bioavailability by preventing precipitation and improving nutrient uptake efficiency, whereas natural chelating agents like humic acids and amino acid complexes offer environmentally friendly alternatives with slower nutrient release and reduced risk of soil contamination. The choice between synthetic and natural chelators influences crop yield and soil health, with synthetic options favored for rapid correction of deficiencies and natural agents preferred for sustainable, long-term soil fertility management.
Chelated micronutrients vs Inorganic salts for plant nutrition Infographic
 agridif.com
agridif.com