Genomic selection significantly enhances the accuracy and speed of breeding for complex traits by utilizing genome-wide marker data to predict breeding values without requiring phenotypic evaluation in every generation. Traditional breeding relies heavily on phenotypic selection and often struggles with traits controlled by many genes and environmental interactions, making progress slower and less predictable. Integrating genomic selection into breeding programs accelerates genetic gain and improves the efficiency of developing superior plant varieties for complex traits such as yield, drought tolerance, and disease resistance.
Table of Comparison
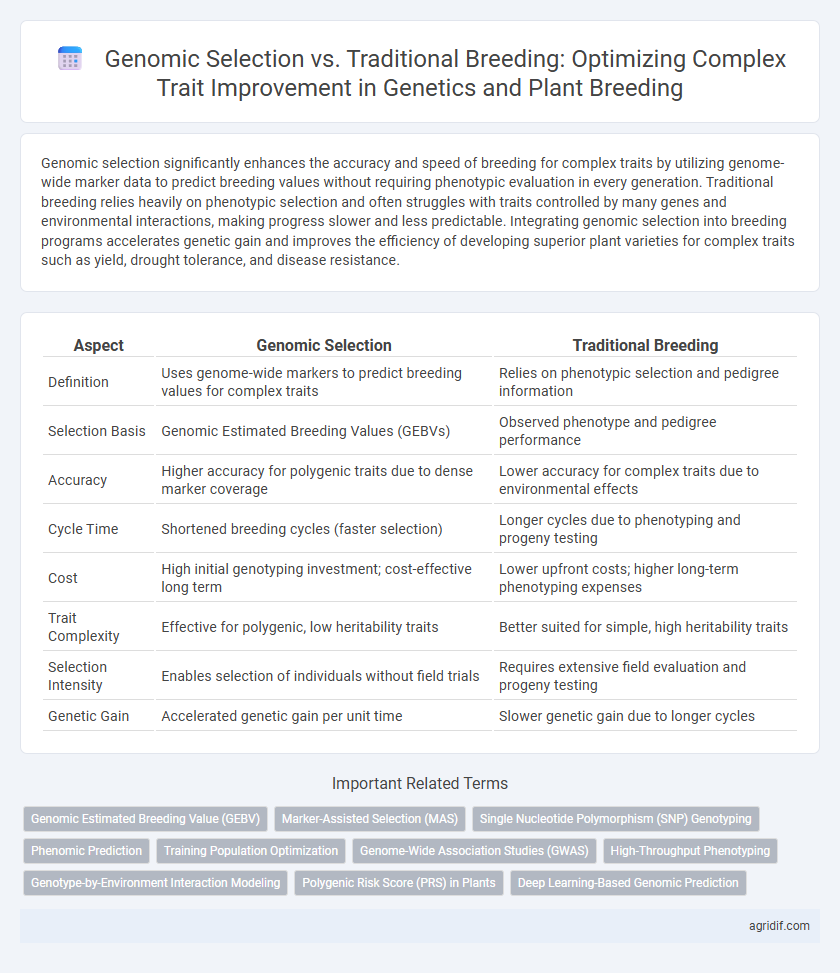
| Aspect | Genomic Selection | Traditional Breeding |
|---|---|---|
| Definition | Uses genome-wide markers to predict breeding values for complex traits | Relies on phenotypic selection and pedigree information |
| Selection Basis | Genomic Estimated Breeding Values (GEBVs) | Observed phenotype and pedigree performance |
| Accuracy | Higher accuracy for polygenic traits due to dense marker coverage | Lower accuracy for complex traits due to environmental effects |
| Cycle Time | Shortened breeding cycles (faster selection) | Longer cycles due to phenotyping and progeny testing |
| Cost | High initial genotyping investment; cost-effective long term | Lower upfront costs; higher long-term phenotyping expenses |
| Trait Complexity | Effective for polygenic, low heritability traits | Better suited for simple, high heritability traits |
| Selection Intensity | Enables selection of individuals without field trials | Requires extensive field evaluation and progeny testing |
| Genetic Gain | Accelerated genetic gain per unit time | Slower genetic gain due to longer cycles |
Introduction to Genomic Selection and Traditional Breeding
Genomic selection leverages genome-wide marker data to predict the breeding value of plants, enabling faster and more accurate selection for complex traits compared to traditional breeding methods that rely on phenotypic evaluation and pedigree information. Traditional breeding involves crossing selected parents followed by phenotypic assessment over multiple generations, which is time-consuming and less efficient for polygenic traits. Advances in genomic selection models incorporate dense marker panels to capture additive genetic variance, significantly improving genetic gain and accelerating breeding cycles.
Understanding Complex Traits in Crop Improvement
Genomic selection leverages genome-wide marker data to predict breeding values, enabling more accurate and faster improvement of complex traits such as yield, drought tolerance, and disease resistance in crops. Traditional breeding relies on phenotypic selection, which often struggles with low heritability and environmental interactions affecting complex traits. Integrating genomic selection enhances selection efficiency by capturing the genetic architecture underlying complex traits, accelerating crop improvement programs.
Principles of Traditional Breeding Methods
Traditional breeding methods rely on phenotypic selection, where plants with desirable traits are chosen based on observable characteristics across multiple generations. This approach uses heritability estimates and genetic variance to guide selection decisions, often requiring extensive field trials and environmental testing to capture complex traits influenced by multiple genes. Although effective, traditional breeding is time-consuming and less precise for traits governed by polygenic inheritance compared to genomic selection.
Fundamentals of Genomic Selection in Plant Breeding
Genomic selection in plant breeding leverages dense genome-wide marker data to predict the genetic potential of individuals for complex traits, enabling early and accurate selection without phenotypic evaluation. This approach incorporates statistical models, such as GBLUP or Bayesian methods, to capture the additive genetic variance from large marker panels, accelerating genetic gain per unit time. By contrast, traditional breeding relies heavily on phenotypic selection, which is less efficient for traits influenced by many genes and environmental interactions.
Comparative Accuracy in Predicting Complex Traits
Genomic selection leverages genome-wide marker data to predict complex traits with higher accuracy compared to traditional breeding, which relies on phenotypic selection and pedigree information. Studies demonstrate that genomic selection can increase predictive accuracy by 20-50% for traits with low heritability and polygenic inheritance. This enhanced precision accelerates genetic gain and improves the efficiency of breeding programs targeting complex traits like yield, disease resistance, and stress tolerance.
Time Efficiency: Genomic Selection vs Traditional Breeding
Genomic selection significantly reduces the breeding cycle time compared to traditional breeding methods by enabling early and accurate prediction of complex traits through genome-wide marker data. Traditional breeding relies on phenotypic evaluations across multiple generations, often requiring years to advance progenies and identify superior genotypes. By integrating genomic prediction models, breeders accelerate selection decisions, facilitating faster genetic gains in crop improvement programs.
Cost-Effectiveness and Resource Allocation
Genomic selection significantly reduces the cost and time required to develop crops with complex traits by enabling early and accurate prediction of genetic potential, thus optimizing resource allocation. Traditional breeding methods demand extensive multi-generation field trials and phenotyping, increasing labor and financial inputs. Implementing genomic selection streamlines breeding programs, making them more economically viable and efficient for achieving genetic gains in complex traits.
Impact on Genetic Diversity and Crop Adaptability
Genomic selection accelerates breeding cycles by using genome-wide markers to predict complex trait performance, enhancing selection accuracy and genetic gain compared to traditional phenotypic methods. This approach maintains broader genetic diversity by capturing small-effect loci across the genome, reducing the risk of genetic bottlenecks common in conventional selection focusing on major traits. Improved crop adaptability results from the increased genetic variation preserved, enabling better responses to environmental stresses and evolving climates.
Integration of Genomic Selection in Modern Breeding Programs
Genomic selection leverages high-density genetic markers and predictive models to accelerate the breeding cycle, offering superior accuracy over traditional phenotypic selection for complex traits like yield and drought tolerance. Integrating genomic selection into modern breeding programs enhances genetic gain by enabling early selection of superior genotypes and reducing reliance on lengthy field trials. Crop improvement pipelines that combine genomic data with advanced phenotyping platforms optimize resource efficiency and maximize breeding program responsiveness to climate variability and evolving market demands.
Future Prospects and Challenges in Complex Trait Improvement
Genomic selection offers significant advantages over traditional breeding by enabling the prediction of complex trait performance using genome-wide markers, accelerating the breeding cycle and increasing genetic gain. Future prospects include integrating high-throughput phenotyping and advanced machine learning models to enhance prediction accuracy and handle genotype-by-environment interactions effectively. Challenges remain in managing large-scale genomic data, addressing the polygenic nature of traits, and ensuring the affordability and accessibility of genomic technologies for diverse breeding programs.
Related Important Terms
Genomic Estimated Breeding Value (GEBV)
Genomic Estimated Breeding Value (GEBV) leverages dense genome-wide markers to predict complex trait performance, enabling more accurate selection compared to phenotypic evaluations alone in traditional breeding. This approach accelerates genetic gain by reducing breeding cycle time and enhancing the precision of selecting individuals with desirable traits in plant breeding programs.
Marker-Assisted Selection (MAS)
Genomic Selection (GS) outperforms Marker-Assisted Selection (MAS) by utilizing genome-wide markers to predict breeding values for complex traits, enabling more accurate and rapid selection compared to MAS which targets a limited number of known quantitative trait loci (QTLs). While MAS is effective in introgressing major genes, GS integrates thousands of markers capturing small-effect loci, enhancing the genetic gain for polygenic traits such as yield and drought tolerance in plant breeding programs.
Single Nucleotide Polymorphism (SNP) Genotyping
Genomic selection leverages high-density Single Nucleotide Polymorphism (SNP) genotyping to predict complex traits with higher accuracy and faster breeding cycles compared to traditional breeding that relies on phenotypic selection alone. SNP markers enable capturing the genetic architecture of traits, facilitating the selection of superior genotypes even for traits with low heritability and complex inheritance patterns.
Phenomic Prediction
Phenomic prediction leverages high-throughput phenotyping to capture complex trait variability and enhances genomic selection accuracy beyond traditional breeding methods that rely on limited phenotypic data. Integrating multidimensional phenomic data with genomic information accelerates the identification of superior genotypes for complex traits in plant breeding programs.
Training Population Optimization
Training population optimization enhances genomic selection accuracy for complex traits by strategically selecting representative and phenotypically diverse individuals, maximizing genetic variance captured in the model. This targeted approach outperforms traditional breeding methods relying on pedigree information, enabling more precise prediction of breeding values and accelerated genetic gain.
Genome-Wide Association Studies (GWAS)
Genome-wide association studies (GWAS) identify genetic variants linked to complex traits, providing high-resolution markers for genomic selection that accelerates breeding cycles and improves prediction accuracy compared to traditional breeding methods. Leveraging GWAS data enables breeders to capture polygenic effects and enhance selection efficiency for traits controlled by multiple genes, such as yield and stress tolerance.
High-Throughput Phenotyping
Genomic selection leverages high-throughput phenotyping technologies to rapidly capture extensive and precise trait data, enhancing prediction accuracy for complex traits compared to traditional breeding methods that rely on limited manual phenotyping. This integration accelerates genetic gain by enabling selection decisions based on comprehensive genotype-phenotype associations rather than solely on phenotypic observations under field conditions.
Genotype-by-Environment Interaction Modeling
Genomic selection enhances prediction accuracy for complex traits by integrating dense marker data and phenotype information, effectively capturing genotype-by-environment interactions through advanced statistical models like genomic best linear unbiased prediction (GBLUP). Traditional breeding methods often lack the capacity to model these interactions explicitly, limiting their efficiency in selecting genotypes with stable performance across diverse environments.
Polygenic Risk Score (PRS) in Plants
Genomic selection leverages Polygenic Risk Scores (PRS) to predict complex quantitative traits in plants by aggregating the effects of numerous small-effect loci, enabling more accurate and accelerated breeding cycles than traditional phenotype-based methods. This approach improves genetic gain for traits like yield, stress tolerance, and disease resistance by integrating dense molecular marker data and statistical models that capture polygenic variation.
Deep Learning-Based Genomic Prediction
Deep learning-based genomic prediction outperforms traditional breeding techniques by leveraging large-scale genomic data to accurately predict complex traits, accelerating selection cycles. These models capture nonlinear interactions and epistatic effects, enabling more precise and efficient identification of superior genotypes for crop improvement.
Genomic Selection vs Traditional Breeding for Complex Traits Infographic
 agridif.com
agridif.com