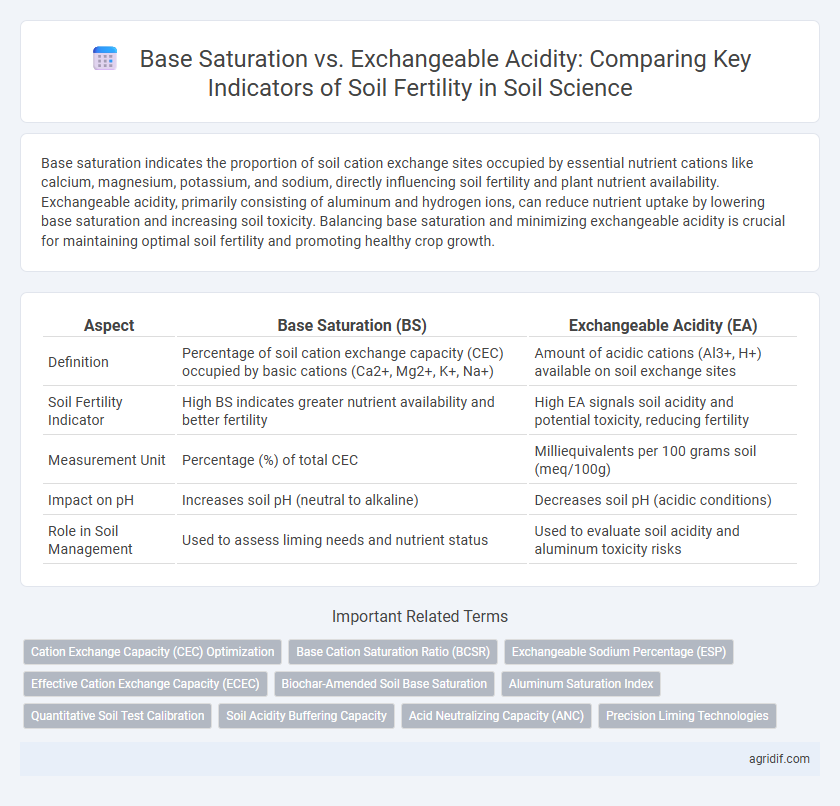
Base saturation indicates the proportion of soil cation exchange sites occupied by essential nutrient cations like calcium, magnesium, potassium, and sodium, directly influencing soil fertility and plant nutrient availability. Exchangeable acidity, primarily consisting of aluminum and hydrogen ions, can reduce nutrient uptake by lowering base saturation and increasing soil toxicity. Balancing base saturation and minimizing exchangeable acidity is crucial for maintaining optimal soil fertility and promoting healthy crop growth.
Table of Comparison
| Aspect | Base Saturation (BS) | Exchangeable Acidity (EA) |
|---|---|---|
| Definition | Percentage of soil cation exchange capacity (CEC) occupied by basic cations (Ca2+, Mg2+, K+, Na+) | Amount of acidic cations (Al3+, H+) available on soil exchange sites |
| Soil Fertility Indicator | High BS indicates greater nutrient availability and better fertility | High EA signals soil acidity and potential toxicity, reducing fertility |
| Measurement Unit | Percentage (%) of total CEC | Milliequivalents per 100 grams soil (meq/100g) |
| Impact on pH | Increases soil pH (neutral to alkaline) | Decreases soil pH (acidic conditions) |
| Role in Soil Management | Used to assess liming needs and nutrient status | Used to evaluate soil acidity and aluminum toxicity risks |
Understanding Base Saturation in Soil Science
Base saturation in soil science represents the proportion of exchangeable bases--calcium, magnesium, potassium, and sodium--relative to the cation exchange capacity (CEC) of the soil, serving as a key indicator of soil fertility. High base saturation levels correlate with increased nutrient availability and optimal pH, promoting plant growth and microbial activity, whereas low base saturation often signals soil acidity and potential nutrient deficiencies. Exchangeable acidity measures the concentration of acidic cations, such as aluminum and hydrogen, which inversely affect base saturation and can impair soil structure and root development.
The Role of Exchangeable Acidity in Soil Fertility
Exchangeable acidity, primarily comprising exchangeable aluminum and hydrogen ions, plays a crucial role in soil fertility by influencing nutrient availability and cation exchange capacity. High levels of exchangeable acidity lower soil pH, leading to aluminum toxicity and nutrient imbalances that adversely affect plant growth. Balancing base saturation and exchangeable acidity is essential to maintain optimal soil conditions for nutrient uptake and sustainable crop production.
Key Differences Between Base Saturation and Exchangeable Acidity
Base saturation represents the proportion of soil cation exchange sites occupied by base cations such as calcium, magnesium, potassium, and sodium, which directly impacts soil nutrient availability and fertility. Exchangeable acidity measures the amount of acidic cations, primarily hydrogen and aluminum ions, that can be displaced from soil particles, influencing soil pH and aluminum toxicity. The key difference lies in base saturation indicating nutrient-rich, fertile conditions, while exchangeable acidity signals potential soil acidity problems inhibiting plant growth.
Impact of Base Saturation on Plant Nutrient Availability
Base saturation significantly influences plant nutrient availability by indicating the proportion of soil exchange sites occupied by essential base cations such as calcium, magnesium, potassium, and sodium. Higher base saturation enhances soil fertility by promoting nutrient-rich conditions that facilitate optimal root absorption and plant growth. Soils with low base saturation often experience increased exchangeable acidity, which can lead to toxic aluminum and hydrogen concentrations, impairing nutrient availability and reducing crop yields.
How Exchangeable Acidity Influences Crop Growth
Exchangeable acidity, comprising primarily of exchangeable aluminum and hydrogen ions, significantly influences crop growth by affecting nutrient availability and root development in acidic soils. High levels of exchangeable acidity reduce base saturation, limiting essential cations such as calcium, magnesium, and potassium, which are critical for plant nutrition. Managing exchangeable acidity through liming improves soil pH, enhances nutrient uptake, and ultimately increases soil fertility and crop yield.
Measuring Base Saturation and Exchangeable Acidity in Soils
Measuring base saturation involves calculating the percentage of the soil's cation exchange capacity (CEC) occupied by basic cations such as calcium, magnesium, potassium, and sodium, which are essential for nutrient availability and soil fertility. Exchangeable acidity is determined by quantifying the concentration of exchangeable hydrogen and aluminum ions using methods like potassium chloride (KCl) extraction, reflecting soil acidity impacting nutrient uptake. Accurate assessment of base saturation and exchangeable acidity provides critical insights into soil pH buffering capacity and guides lime application for optimal crop growth.
Optimal Base Saturation Levels for Major Crops
Optimal base saturation levels vary by crop, with most major crops thriving at 60-80% base saturation to ensure adequate nutrient availability and root health. Low base saturation often correlates with high exchangeable acidity, which can lead to aluminum toxicity and reduced nutrient uptake in crops like maize, wheat, and soybeans. Maintaining base saturation within this optimal range improves cation exchange capacity and soil fertility, promoting higher crop yields and sustainable soil management.
Managing Exchangeable Acidity to Improve Soil Health
Managing exchangeable acidity is crucial for improving soil fertility, as high acidity reduces nutrient availability and disrupts microbial activity in the rhizosphere. Base saturation percentage serves as a key indicator, where increasing base cations like calcium, magnesium, and potassium through liming can effectively neutralize exchangeable aluminum and hydrogen ions. Optimizing base saturation enhances soil structure and nutrient retention, promoting healthier root development and higher crop yields.
Interactions Between Base Saturation and Soil pH
Base saturation, representing the proportion of soil exchange sites occupied by basic cations such as calcium, magnesium, potassium, and sodium, directly influences soil pH by neutralizing exchangeable acidity primarily from hydrogen and aluminum ions. Higher base saturation levels correspond to increased soil pH, enhancing nutrient availability and microbial activity essential for soil fertility. The dynamic balance between base saturation and exchangeable acidity determines soil acidity, thereby regulating nutrient retention and overall soil productivity.
Strategies for Balancing Base Saturation and Reducing Acidity
Balancing base saturation and reducing exchangeable acidity in soil fertility involves applying lime amendments to increase soil pH and neutralize toxic aluminum ions, enhancing nutrient availability for plants. Integrating organic matter such as compost improves cation exchange capacity, promoting retention of essential base cations like calcium, magnesium, and potassium. Regular soil testing guides precise liming and nutrient management, optimizing base saturation levels while minimizing exchangeable acidity to sustain crop productivity.
Related Important Terms
Cation Exchange Capacity (CEC) Optimization
Base saturation directly influences soil fertility by indicating the proportion of nutrient-rich cations such as calcium, magnesium, potassium, and sodium occupying the Cation Exchange Capacity (CEC), whereas exchangeable acidity represents hydrogen and aluminum ions that reduce nutrient availability. Optimizing CEC through balanced base saturation and minimizing exchangeable acidity enhances nutrient retention and promotes optimal plant growth in diverse soil types.
Base Cation Saturation Ratio (BCSR)
Base Cation Saturation Ratio (BCSR) quantifies the proportion of essential base cations (Ca2+, Mg2+, K+, Na+) relative to total cation exchange capacity (CEC), directly influencing soil fertility by balancing nutrient availability and mitigating exchangeable acidity. Optimal BCSR maintains base saturation above 70%, minimizing aluminum and hydrogen ions that cause soil acidity, thereby enhancing root growth and microbial activity essential for nutrient cycling.
Exchangeable Sodium Percentage (ESP)
Exchangeable Sodium Percentage (ESP) directly influences soil structure and fertility by affecting base saturation levels and increasing exchangeable acidity, leading to reduced nutrient availability and impaired soil permeability. High ESP values contribute to soil alkalinity and sodicity issues, which diminish crop productivity by disrupting cation balance and soil chemical properties.
Effective Cation Exchange Capacity (ECEC)
Base Saturation represents the proportion of the Effective Cation Exchange Capacity (ECEC) occupied by essential nutrient cations such as calcium, magnesium, potassium, and sodium, indicating soil fertility status. Exchangeable Acidity, primarily consisting of hydrogen and aluminum ions on the ECEC sites, reflects soil acidity that can limit nutrient availability and negatively impact crop growth.
Biochar-Amended Soil Base Saturation
Base saturation in biochar-amended soils increases nutrient availability by occupying exchange sites with basic cations, thereby reducing exchangeable acidity and enhancing soil fertility. High base saturation correlates with improved cation exchange capacity and pH stabilization, promoting optimal nutrient uptake for plant growth.
Aluminum Saturation Index
Base Saturation percentage measures the proportion of soil exchange sites occupied by essential cations like calcium, magnesium, potassium, and sodium, directly impacting soil fertility and nutrient availability. The Aluminum Saturation Index, indicating the fraction of exchangeable acidity represented by toxic aluminum ions, serves as a critical factor in assessing soil acidity and potential aluminum toxicity risks to plant roots.
Quantitative Soil Test Calibration
Base saturation percentage directly influences soil fertility by indicating the proportion of exchangeable cations such as calcium, magnesium, potassium, and sodium relative to the cation exchange capacity, while exchangeable acidity measures the concentration of exchangeable aluminum and hydrogen ions that can inhibit nutrient availability. Quantitative soil test calibration correlates base saturation levels above 60% with optimal nutrient availability and reduced exchangeable acidity, facilitating precise lime application rates for effective soil pH adjustment and enhanced crop yield.
Soil Acidity Buffering Capacity
Base saturation indicates the proportion of soil cation exchange capacity occupied by basic cations, directly influencing nutrient availability and soil fertility. Exchangeable acidity, primarily from Al3+ and H+ ions, reflects soil acidity buffering capacity, crucial for maintaining pH stability and the effective management of acid-sensitive crops.
Acid Neutralizing Capacity (ANC)
Base saturation represents the proportion of soil cation exchange sites occupied by basic cations (Ca2+, Mg2+, K+, Na+), directly correlating with the soil's Acid Neutralizing Capacity (ANC) and overall fertility potential. Exchangeable acidity, primarily from aluminum and hydrogen ions, inversely affects ANC by increasing soil acidity, thus reducing nutrient availability and impairing crop productivity.
Precision Liming Technologies
Base saturation directly influences soil fertility by indicating the proportion of exchangeable bases like calcium, magnesium, potassium, and sodium, which are essential for nutrient availability and crop growth, whereas exchangeable acidity reflects the concentration of acidic cations such as hydrogen and aluminum ions that can harm root development. Precision liming technologies enhance soil fertility management by accurately measuring and adjusting base saturation and exchangeable acidity levels, ensuring optimal pH balance and nutrient availability tailored to specific soil conditions.
Base Saturation vs Exchangeable Acidity for Soil Fertility Infographic
 agridif.com
agridif.com