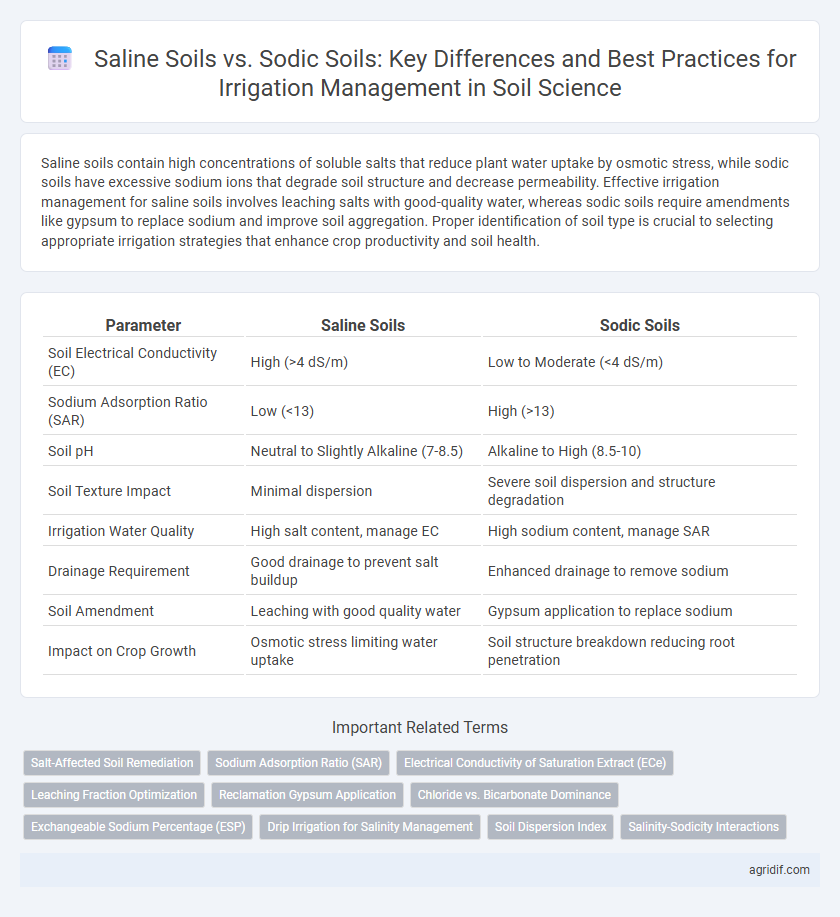
Saline soils contain high concentrations of soluble salts that reduce plant water uptake by osmotic stress, while sodic soils have excessive sodium ions that degrade soil structure and decrease permeability. Effective irrigation management for saline soils involves leaching salts with good-quality water, whereas sodic soils require amendments like gypsum to replace sodium and improve soil aggregation. Proper identification of soil type is crucial to selecting appropriate irrigation strategies that enhance crop productivity and soil health.
Table of Comparison
| Parameter | Saline Soils | Sodic Soils |
|---|---|---|
| Soil Electrical Conductivity (EC) | High (>4 dS/m) | Low to Moderate (<4 dS/m) |
| Sodium Adsorption Ratio (SAR) | Low (<13) | High (>13) |
| Soil pH | Neutral to Slightly Alkaline (7-8.5) | Alkaline to High (8.5-10) |
| Soil Texture Impact | Minimal dispersion | Severe soil dispersion and structure degradation |
| Irrigation Water Quality | High salt content, manage EC | High sodium content, manage SAR |
| Drainage Requirement | Good drainage to prevent salt buildup | Enhanced drainage to remove sodium |
| Soil Amendment | Leaching with good quality water | Gypsum application to replace sodium |
| Impact on Crop Growth | Osmotic stress limiting water uptake | Soil structure breakdown reducing root penetration |
Understanding Saline and Sodic Soils: Key Definitions
Saline soils contain high concentrations of soluble salts, primarily sodium chloride, which increase soil salinity and affect plant water uptake during irrigation. Sodic soils are characterized by high sodium adsorption ratios (SAR), leading to soil dispersion, poor structure, and reduced permeability, which complicate irrigation efficiency. Proper identification of saline versus sodic soils through electrical conductivity (EC) and sodium adsorption ratio (SAR) measurements is essential for effective irrigation management and soil reclamation strategies.
Causes and Formation of Saline vs Sodic Soils
Saline soils form primarily due to the accumulation of soluble salts like sodium chloride, calcium sulfate, and magnesium sulfate, often resulting from high evaporation rates and poor drainage in arid and semi-arid regions. Sodic soils develop due to excessive sodium ion concentration on soil exchange sites, often caused by irrigation with sodium-rich water or natural weathering of minerals, leading to dispersion of soil particles and poor soil structure. The contrasting chemical properties influence irrigation management strategies, where saline soils require leaching to remove salts, while sodic soils need amendments like gypsum to replace sodium ions and improve soil permeability.
Physical and Chemical Properties Analysis
Saline soils contain high concentrations of soluble salts, primarily sodium chloride, which increase electrical conductivity and osmotic pressure, affecting water availability for plants. Sodic soils exhibit elevated sodium levels, leading to soil structure degradation through dispersion of clay particles, reduced permeability, and poor aeration, impairing root growth. Effective irrigation management requires precise analysis of soil pH, sodium adsorption ratio (SAR), and exchangeable sodium percentage (ESP) to tailor reclamation practices and optimize water infiltration and crop productivity.
Impacts on Crop Growth and Yield
Saline soils contain high concentrations of soluble salts that reduce water availability to crops, leading to osmotic stress and decreased yield. Sodic soils, characterized by excessive sodium ions, cause soil structure deterioration, poor aeration, and impaired root penetration, significantly limiting crop growth. Effective irrigation management requires distinguishing between these soil types to apply appropriate amendments, such as gypsum for sodic soils and leaching strategies for saline soils, to optimize crop productivity.
Water Quality and Its Role in Soil Salinity and Sodicity
Water quality plays a crucial role in managing saline and sodic soils during irrigation, with saline soils characterized by high concentrations of soluble salts affecting osmotic potential and sodic soils dominated by excessive sodium ions disrupting soil structure. Irrigation water with high electrical conductivity (EC) increases soil salinity, while elevated sodium adsorption ratio (SAR) in water promotes sodicity, leading to poor infiltration and permeability. Effective irrigation management involves selecting water sources with optimal EC and SAR values to prevent soil degradation, enhance crop productivity, and maintain soil health.
Diagnostic Techniques for Soil Salinity and Sodicity
Soil salinity is identified through electrical conductivity (EC) measurements of the soil solution, where values exceeding 4 dS/m typically indicate saline soils, while sodic soils are diagnosed by measuring exchangeable sodium percentage (ESP) above 15%. Soil sampling followed by laboratory analysis of pH, EC, and sodium adsorption ratio (SAR) provides precise differentiation between saline and sodic conditions critical for irrigation management. Field methods such as soil paste tests and on-site EC meters complement laboratory diagnostics, enabling timely identification and treatment planning for soil salinity and sodicity issues.
Irrigation Strategies for Saline Soils
Irrigation strategies for saline soils prioritize the application of high-quality water with low salt content to prevent further salt accumulation in the root zone. Leaching with excess water beyond crop evapotranspiration effectively flushes salts below the root zone, improving soil salinity levels and maintaining crop productivity. Monitoring soil electrical conductivity (EC) regularly guides irrigation scheduling and helps optimize water use for sustainable management of saline soils.
Best Irrigation Practices for Sodic Soils
Sodic soils, characterized by high sodium content and poor soil structure, require specific irrigation practices to prevent waterlogging and enhance permeability. Using gypsum (calcium sulfate) as an amendment combined with controlled leaching irrigation promotes sodium displacement and improves soil aggregation. Maintaining proper irrigation scheduling, avoiding excessive water application, and monitoring electrical conductivity (EC) help optimize salinity and sodicity levels for sustainable crop production.
Remediation and Reclamation Approaches
Saline soils contain high soluble salts which impair water uptake by plants, and reclamation involves leaching with good quality water combined with proper drainage to prevent salt accumulation. Sodic soils have high sodium content causing soil dispersion and poor structure, requiring amendments like gypsum to replace sodium ions and improve soil permeability before leaching. Effective irrigation management integrates soil testing, application of chemical amendments, and controlled water application to restore soil health and optimize crop productivity.
Monitoring and Long-term Management Recommendations
Saline soils, characterized by high soluble salt concentrations, require continuous monitoring of electrical conductivity (EC) levels to prevent salt accumulation that impairs plant water uptake. Sodic soils, identified by elevated sodium adsorption ratio (SAR) and poor soil structure, demand regular assessment of exchangeable sodium percentage (ESP) and amendments with gypsum to restore soil permeability. Long-term irrigation management strategies should incorporate leaching requirements, proper water quality evaluation, and periodic soil testing to maintain optimal soil health and crop productivity.
Related Important Terms
Salt-Affected Soil Remediation
Saline soils contain high concentrations of soluble salts causing osmotic stress to plants, while sodic soils have excessive sodium ions that degrade soil structure and reduce permeability, demanding distinct remediation approaches for irrigation management. Effective salt-affected soil remediation involves leaching saline salts with high-quality water and employing gypsum or other calcium amendments to displace sodium in sodic soils, thereby restoring soil permeability and fertility.
Sodium Adsorption Ratio (SAR)
Saline soils contain high concentrations of soluble salts that impact water availability, while sodic soils are characterized by a high Sodium Adsorption Ratio (SAR), indicating excessive sodium relative to calcium and magnesium, which adversely affects soil structure and permeability. Effective irrigation management requires monitoring SAR to prevent sodium buildup, ensuring proper soil dispersion and maintaining water infiltration rates.
Electrical Conductivity of Saturation Extract (ECe)
Saline soils exhibit high Electrical Conductivity of Saturation Extract (ECe) values, typically above 4 dS/m, indicating elevated soluble salt concentrations that can reduce plant water uptake during irrigation. In contrast, sodic soils may have low or normal ECe values but are characterized by high sodium adsorption ratios (SAR), leading to soil dispersion and poor infiltration regardless of electrical conductivity levels.
Leaching Fraction Optimization
Saline soils contain high concentrations of soluble salts that reduce osmotic potential, requiring precise leaching fraction optimization to prevent salt accumulation during irrigation. Sodic soils, characterized by high sodium levels disrupting soil structure, demand tailored leaching fractions to enhance sodium displacement and maintain soil permeability for effective crop growth.
Reclamation Gypsum Application
Saline soils contain high soluble salts that reduce osmotic potential, while sodic soils have excessive sodium ions causing soil dispersion and poor structure; reclamation of sodic soils effectively requires gypsum application to replace sodium with calcium ions, improving soil permeability and water infiltration. Gypsum application enhances soil aggregation and facilitates leaching of sodium salts during irrigation, crucial for restoring sodic soil fertility and achieving sustainable crop production.
Chloride vs. Bicarbonate Dominance
Saline soils are characterized by high concentrations of chloride ions, which can cause osmotic stress and reduce plant water uptake during irrigation. Sodic soils predominantly contain bicarbonate ions, leading to soil dispersion, poor structure, and reduced permeability, necessitating specific amendments for effective irrigation management.
Exchangeable Sodium Percentage (ESP)
Saline soils, characterized by high soluble salt concentration but low Exchangeable Sodium Percentage (ESP below 15%), generally pose less risk to soil structure during irrigation, whereas sodic soils exhibit high ESP values (above 15%), leading to soil dispersion, reduced permeability, and poor water infiltration. Effective irrigation management requires monitoring ESP levels to prevent sodium-induced soil degradation, often necessitating gypsum application or leaching practices to replace sodium ions with calcium and maintain soil health.
Drip Irrigation for Salinity Management
Saline soils contain high concentrations of soluble salts that reduce crop water uptake, while sodic soils have excessive sodium ions that degrade soil structure and permeability, complicating irrigation management. Drip irrigation effectively manages salinity by delivering precise water amounts directly to the root zone, minimizing salt accumulation on the soil surface and preventing further salinization.
Soil Dispersion Index
Saline soils, characterized by high soluble salt concentrations, typically exhibit low soil dispersion index values, making them less prone to structural breakdown during irrigation, whereas sodic soils, dominated by exchangeable sodium ions, show high dispersion index values, leading to poor soil aggregation and increased erosion risk. Effective irrigation management requires accurate assessment of the soil dispersion index to prevent soil dispersion, maintain permeability, and preserve soil structure in sodic conditions.
Salinity-Sodicity Interactions
Saline soils contain high concentrations of soluble salts that reduce water availability and affect crop yield, while sodic soils are characterized by elevated sodium levels that deteriorate soil structure and permeability. The interaction between salinity and sodicity complicates irrigation management by influencing soil infiltration, nutrient balance, and plant stress tolerance, requiring tailored water quality and amendment strategies to restore soil health and optimize crop production.
Saline Soils vs Sodic Soils for Irrigation Management Infographic
 agridif.com
agridif.com