Soil pH significantly influences cation exchange capacity (CEC) by affecting the charge and activity of soil colloids, which in turn dictates nutrient availability to plants. Soils with optimal pH levels enhance CEC, promoting the retention and exchange of essential nutrients like calcium, magnesium, and potassium. Low or high pH can reduce CEC effectiveness, limiting nutrient accessibility and impacting overall soil fertility.
Table of Comparison
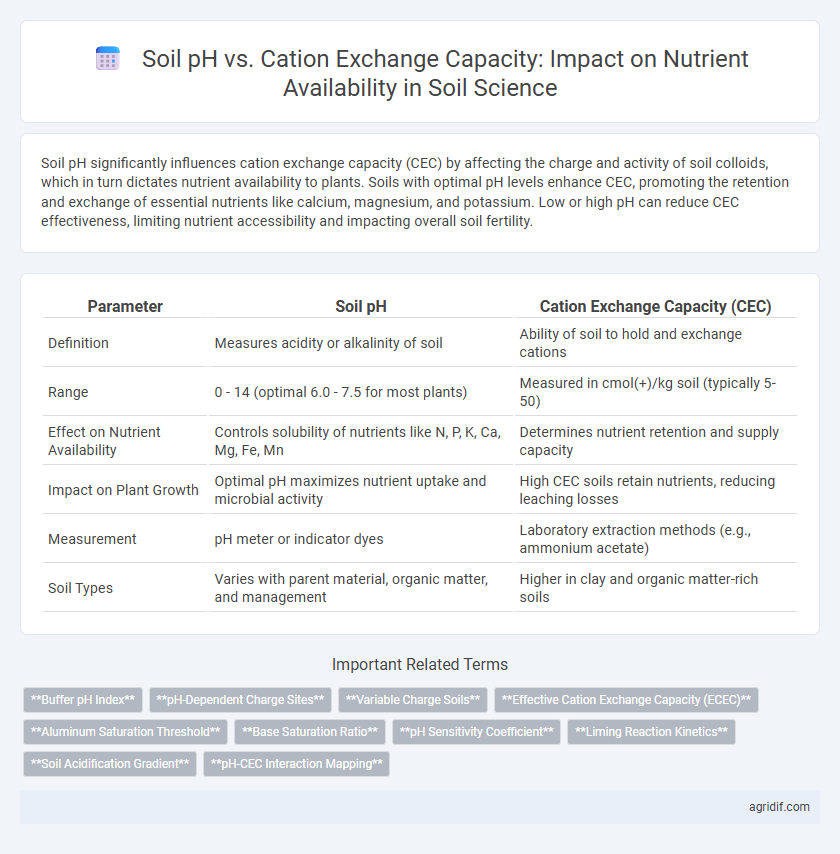
| Parameter | Soil pH | Cation Exchange Capacity (CEC) |
|---|---|---|
| Definition | Measures acidity or alkalinity of soil | Ability of soil to hold and exchange cations |
| Range | 0 - 14 (optimal 6.0 - 7.5 for most plants) | Measured in cmol(+)/kg soil (typically 5-50) |
| Effect on Nutrient Availability | Controls solubility of nutrients like N, P, K, Ca, Mg, Fe, Mn | Determines nutrient retention and supply capacity |
| Impact on Plant Growth | Optimal pH maximizes nutrient uptake and microbial activity | High CEC soils retain nutrients, reducing leaching losses |
| Measurement | pH meter or indicator dyes | Laboratory extraction methods (e.g., ammonium acetate) |
| Soil Types | Varies with parent material, organic matter, and management | Higher in clay and organic matter-rich soils |
Understanding Soil pH: Definition and Importance
Soil pH measures the acidity or alkalinity of soil, influencing nutrient solubility and microbial activity essential for plant growth. Optimal soil pH typically ranges from 6.0 to 7.5, where cation exchange capacity (CEC) is most effective in retaining essential nutrients such as calcium, magnesium, and potassium. Understanding soil pH is critical for managing nutrient availability, optimizing fertilizer use, and promoting healthy soil biology.
What is Cation Exchange Capacity (CEC) in Soils?
Cation Exchange Capacity (CEC) in soils is a measure of the soil's ability to hold and exchange positively charged ions (cations) such as calcium, magnesium, potassium, and sodium, which are essential for plant nutrient uptake. Higher CEC soils can retain more nutrients, improving nutrient availability and reducing leaching losses. Soil pH influences CEC by affecting the charge characteristics of soil particles and organic matter, thereby impacting nutrient retention and availability.
The Role of Soil pH in Nutrient Availability
Soil pH critically influences nutrient availability by affecting the solubility of minerals and the cation exchange capacity (CEC) of soil particles. At optimal pH levels (typically between 6 and 7), essential nutrients like nitrogen, phosphorus, and potassium remain more soluble and accessible to plant roots, while extreme pH values can lock nutrients in unavailable forms. The interaction between soil pH and CEC dictates the soil's ability to retain and supply base cations such as calcium, magnesium, and potassium, directly impacting plant nutrition and growth.
How Cation Exchange Capacity Influences Soil Fertility
Cation Exchange Capacity (CEC) directly affects soil fertility by regulating the soil's ability to retain and supply essential nutrients such as calcium, magnesium, and potassium to plants. Soils with high CEC have greater nutrient-holding capacity, reducing nutrient leaching and improving nutrient availability. Optimal soil pH enhances CEC efficiency, as nutrient ions are more exchangeable and accessible to plant roots within a pH range that supports microbial activity and nutrient solubility.
Interaction Between Soil pH and CEC
Soil pH significantly influences Cation Exchange Capacity (CEC) by affecting the ionization state of soil colloids, thereby altering nutrient retention and availability. At optimal pH levels, typically between 6 and 7, CEC is maximized, enhancing the soil's ability to hold essential cations such as calcium, magnesium, and potassium for plant uptake. Acidic or alkaline conditions reduce CEC efficiency, leading to nutrient imbalances and decreased soil fertility.
Soil pH Effects on Cation Exchange Processes
Soil pH significantly influences cation exchange capacity (CEC) by affecting the charge and availability of exchange sites on soil particles, which directly impacts nutrient retention and availability. At lower pH levels, increased hydrogen ion concentration can displace essential nutrient cations such as calcium, magnesium, and potassium from exchange sites, reducing their accessibility to plants. Optimal pH levels enhance CEC by maintaining a balanced negative charge on soil colloids, facilitating effective nutrient exchange and uptake critical for plant growth.
Factors Affecting Soil CEC and pH
Soil pH directly influences Cation Exchange Capacity (CEC) by affecting the ionization state of soil colloids and the solubility of nutrient cations such as calcium, magnesium, and potassium. Factors affecting soil CEC include soil texture, organic matter content, and mineral composition, with clay and organic matter typically increasing CEC due to their high negative charge. Soil pH also impacts nutrient availability by altering the chemical forms of nutrients, making certain essential elements more accessible or toxic depending on the pH range.
Crop Performance: Soil pH vs CEC Impacts
Soil pH directly influences nutrient solubility and availability, affecting crop nutrient uptake and growth efficiency. Cation Exchange Capacity (CEC) measures soil's ability to retain essential nutrient ions, impacting long-term fertility and crop yield stability. Optimal crop performance is achieved when soil pH is balanced with high CEC, enhancing nutrient retention and reducing leaching losses.
Managing Soil pH and CEC for Optimal Nutrient Uptake
Soil pH critically influences cation exchange capacity (CEC), affecting nutrient availability and plant uptake efficiency. Managing soil pH within an optimal range of 6.0 to 7.0 maximizes CEC, enhancing the soil's ability to retain essential nutrients like calcium, magnesium, and potassium. Adjusting pH through lime application or acidifying amendments ensures balanced nutrient exchange, improving crop productivity and soil health.
Best Practices for Balancing Soil pH and Cation Exchange Capacity
Balancing soil pH between 6.0 and 7.0 optimizes nutrient availability by enhancing cation exchange capacity (CEC), which facilitates the retention and exchange of essential nutrients like calcium, magnesium, and potassium. Regular soil testing enables precise amendments, such as liming acidic soils to raise pH and improve CEC, or applying sulfur to lower alkaline soil pH and prevent nutrient lock-up. Incorporating organic matter increases CEC by providing additional negatively charged sites that hold nutrients, improving overall soil fertility and plant nutrient uptake efficiency.
Related Important Terms
Buffer pH Index
Buffer pH Index is a critical parameter that quantifies soil's resistance to pH changes, directly influencing Cation Exchange Capacity (CEC) and nutrient availability by maintaining stable ionic balance in the root zone. Soils with optimal Buffer pH Index enhance nutrient retention and prevent toxicities, maximizing crop uptake efficiency through improved CEC interactions.
pH-Dependent Charge Sites
Soil pH directly influences the pH-dependent charge sites on soil colloids, which regulate the cation exchange capacity (CEC) and thereby affect nutrient availability. At lower pH levels, increased positive charge reduces CEC by neutralizing negative sites, while higher pH enhances negative charge sites, promoting greater retention of essential cations like calcium, magnesium, and potassium for plant uptake.
Variable Charge Soils
Variable charge soils exhibit pH-dependent cation exchange capacity (CEC), where acidic conditions increase positive charge sites, reducing overall nutrient retention, while alkaline conditions enhance negative charge sites, promoting cation adsorption critical for nutrient availability. This dynamic pH-CEC relationship in variable charge soils directly impacts the bioavailability of essential nutrients like calcium, magnesium, and potassium, influencing soil fertility and crop productivity.
Effective Cation Exchange Capacity (ECEC)
Effective Cation Exchange Capacity (ECEC) critically influences nutrient availability by regulating the soil's ability to retain essential cations such as calcium, magnesium, and potassium, particularly in acidic soils with low pH levels. Optimal ECEC values enhance nutrient retention and reduce leaching, thereby improving soil fertility and plant nutrient uptake efficiency.
Aluminum Saturation Threshold
Soil pH critically influences cation exchange capacity (CEC) by affecting aluminum saturation thresholds, where elevated aluminum ions at low pH levels reduce nutrient availability and impair root growth. Maintaining soil pH above the aluminum saturation threshold--typically around 60% aluminum saturation--ensures optimal CEC function and nutrient exchange efficiency for plant health.
Base Saturation Ratio
Base saturation ratio, the proportion of exchangeable bases (Ca2+, Mg2+, K+, Na+) relative to the soil's cation exchange capacity (CEC), directly influences nutrient availability by regulating soil pH and nutrient retention. Optimal base saturation ratios enhance nutrient uptake efficiency, whereas imbalances may lead to nutrient deficiencies or toxicities affecting plant growth.
pH Sensitivity Coefficient
The pH sensitivity coefficient quantifies the impact of soil pH fluctuations on cation exchange capacity (CEC), directly influencing nutrient availability by altering the soil's ability to retain essential cations such as calcium, magnesium, and potassium. Accurate assessment of this coefficient is critical for optimizing soil amendments to maintain ideal pH ranges that maximize nutrient retention and plant uptake efficiency.
Liming Reaction Kinetics
Liming reaction kinetics directly influence soil pH by neutralizing acidity and subsequently enhancing cation exchange capacity (CEC), which increases the soil's ability to retain essential nutrients such as calcium, magnesium, and potassium. Faster liming reactions improve nutrient availability by accelerating pH adjustment, thereby optimizing microbial activity and nutrient uptake in acid-affected soils.
Soil Acidification Gradient
Soil acidification gradient significantly influences the relationship between soil pH and cation exchange capacity (CEC), where lower pH in acidic soils reduces CEC by increasing hydrogen and aluminum ion dominance, thus limiting nutrient availability. As soil pH declines from neutral to acidic, essential nutrients like calcium, magnesium, and potassium become less available due to decreased CEC and increased leaching losses.
pH-CEC Interaction Mapping
Soil pH directly influences Cation Exchange Capacity (CEC) by affecting the ionization of soil colloids, where acidic soils (pH < 5.5) lower CEC due to proton saturation, while neutral to slightly alkaline soils (pH 6.5-7.5) enhance CEC and nutrient retention. Mapping pH-CEC interactions enables precise adjustment of soil amendments to optimize nutrient availability, particularly for essential cations like Ca2+, Mg2+, and K+, in variable soil types.
Soil pH vs Cation Exchange Capacity for Nutrient Availability Infographic
 agridif.com
agridif.com