Chelated micronutrients offer enhanced stability and bioavailability compared to inorganic salts, ensuring more efficient uptake by plants in agrochemical applications. Their ability to prevent nutrient precipitation and reduce soil fixation leads to improved nutrient delivery and sustained plant growth. In contrast, inorganic salts often face challenges of rapid leaching and lower absorption rates, impacting crop yield and quality.
Table of Comparison
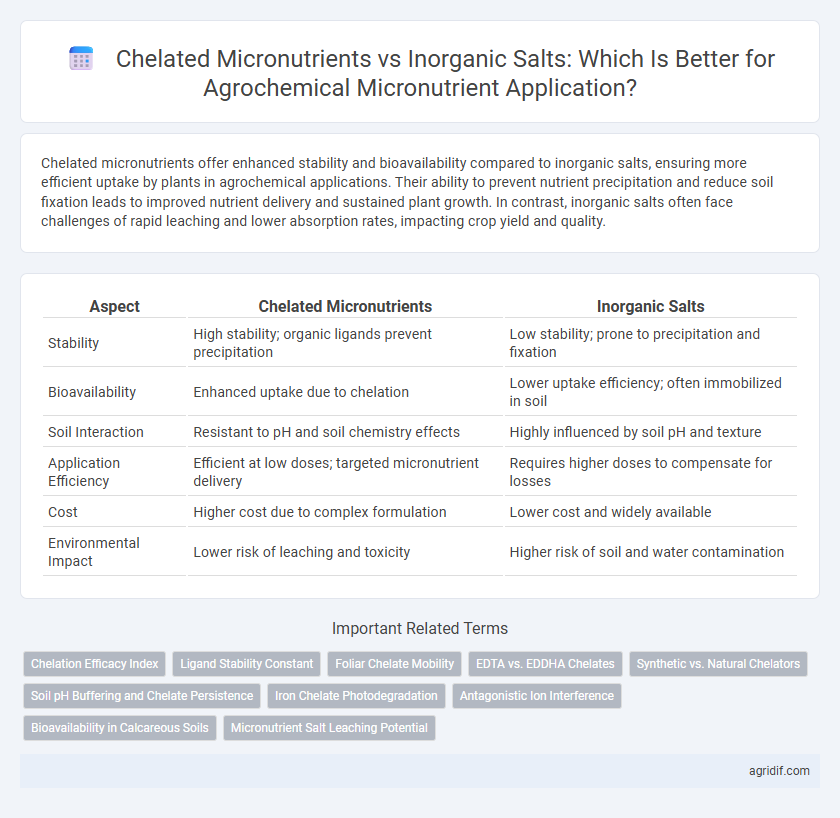
| Aspect | Chelated Micronutrients | Inorganic Salts |
|---|---|---|
| Stability | High stability; organic ligands prevent precipitation | Low stability; prone to precipitation and fixation |
| Bioavailability | Enhanced uptake due to chelation | Lower uptake efficiency; often immobilized in soil |
| Soil Interaction | Resistant to pH and soil chemistry effects | Highly influenced by soil pH and texture |
| Application Efficiency | Efficient at low doses; targeted micronutrient delivery | Requires higher doses to compensate for losses |
| Cost | Higher cost due to complex formulation | Lower cost and widely available |
| Environmental Impact | Lower risk of leaching and toxicity | Higher risk of soil and water contamination |
Introduction to Micronutrient Application in Agriculture
Chelated micronutrients enhance nutrient availability by stabilizing essential metals like iron, zinc, and manganese, preventing precipitation and adsorption in soil, unlike inorganic salts that often suffer from rapid fixation and reduced uptake. The chelation process enables better mobility and controlled release, improving plant absorption efficiency and crop yield quality. Effective micronutrient application using chelated forms addresses soil deficiencies and optimizes nutrient use efficiency, critical for sustainable agricultural productivity.
Understanding Chelated Micronutrients
Chelated micronutrients improve nutrient availability and uptake in plants by forming stable complexes with organic molecules, which prevent precipitation and reduce soil fixation compared to inorganic salts. These chelates enhance leaf absorption and mobility of essential elements like iron, zinc, and manganese, resulting in more efficient correction of micronutrient deficiencies. The superior stability constant and targeted delivery of chelated forms increase bioavailability and reduce environmental losses, making them highly effective in agrochemical formulations.
Characteristics of Inorganic Salt Micronutrients
Inorganic salt micronutrients, commonly used in agrochemical formulations, exhibit high solubility in water, enabling rapid availability to plants but are prone to leaching and fixation in soil matrices. These salts often have simpler chemical structures, which can result in lower stability under varying pH and redox conditions, reducing their efficacy during foliar or soil application. Their cost-effectiveness and ease of production make them widely used, although they may require careful management to avoid micronutrient imbalances or phytotoxicity in crops.
Comparative Bioavailability: Chelates vs Inorganic Salts
Chelated micronutrients exhibit superior bioavailability compared to inorganic salts due to their stable molecular structure, which prevents rapid precipitation and enhances nutrient uptake efficiency in plants. Inorganic salts often face challenges like soil pH interactions and fixation, reducing micronutrient availability and leading to lower absorption rates. Studies show that chelated forms significantly improve nutrient mobility and facilitate sustained release, optimizing plant growth and yield.
Soil Interaction and Mobility Differences
Chelated micronutrients exhibit enhanced soil interaction and mobility compared to inorganic salts due to their stable complex formation with organic ligands, which prevents precipitation and fixation in the soil. Inorganic salts often undergo rapid adsorption, precipitation, or conversion to insoluble forms, reducing nutrient availability to plants. This difference in behavior leads to improved bioavailability and efficient uptake of micronutrients when applied as chelates in diverse soil pH conditions.
Plant Uptake Efficiency: Chelated vs Inorganic Forms
Chelated micronutrients exhibit higher plant uptake efficiency compared to inorganic salts due to their stable molecular structure that prevents nutrient precipitation and enhances bioavailability in the rhizosphere. Inorganic salts often undergo rapid conversion to insoluble forms, limiting nutrient absorption and resulting in lower translocation within plant tissues. Consequently, chelated forms ensure sustained micronutrient availability, improving plant growth and yield in diverse soil conditions.
Impact on Crop Yield and Quality
Chelated micronutrients enhance crop yield and quality more effectively than inorganic salts by improving nutrient uptake efficiency and stability in soil. These chelates reduce micronutrient fixation and leaching, resulting in consistent availability to plants during critical growth stages. Increased bioavailability of chelated elements like iron, zinc, and manganese directly contributes to higher photosynthesis rates, improved fruit coloration, and overall crop vitality.
Compatibility with Fertigation and Foliar Application
Chelated micronutrients exhibit superior compatibility with fertigation and foliar application compared to inorganic salts due to their enhanced solubility and stability across a broad pH range, reducing precipitation and nutrient antagonism. Their molecular structure allows efficient absorption through leaf cuticles and root systems, maximizing nutrient uptake and minimizing losses. In contrast, inorganic salts often face limitations like rapid precipitation, lower bioavailability, and increased risk of leaf phytotoxicity, undermining their effectiveness in precision agriculture practices.
Environmental Considerations and Residual Effects
Chelated micronutrients improve environmental compatibility by reducing nutrient runoff and leaching compared to inorganic salts, which often lead to soil and water contamination. Their controlled-release properties minimize residual toxicity, enhancing soil health and reducing the accumulation of heavy metals in ecosystems. Choosing chelated forms supports sustainable agriculture through better nutrient use efficiency and lower environmental impact.
Economic Analysis: Cost-Effectiveness of Chelates vs Inorganic Salts
Chelated micronutrients demonstrate higher cost-effectiveness compared to inorganic salts due to improved nutrient uptake efficiency and reduced application rates. Although chelates have a higher initial price per unit, their enhanced bioavailability lowers overall input costs by minimizing the need for repeat treatments and reducing crop losses from nutrient deficiencies. Economic analysis consistently shows that the long-term yield gains and soil health benefits of chelated micronutrients outweigh the upfront savings associated with inorganic salt applications.
Related Important Terms
Chelation Efficacy Index
Chelated micronutrients exhibit a higher Chelation Efficacy Index compared to inorganic salts, enhancing nutrient stability and bioavailability in agrochemical applications. This increased chelation efficiency reduces nutrient fixation in soil, promoting better plant uptake and improved crop yield.
Ligand Stability Constant
Chelated micronutrients exhibit higher ligand stability constants compared to inorganic salts, enhancing nutrient availability and uptake efficiency in plants. This increased stability prevents premature micronutrient precipitation and oxidation, ensuring sustained nutrient release and improved agrochemical performance.
Foliar Chelate Mobility
Foliar chelated micronutrients demonstrate enhanced mobility within plant tissues compared to inorganic salts, enabling more efficient nutrient translocation and improved uptake efficiency. The stability of chelates prevents premature nutrient precipitation, ensuring sustained availability and minimizing nutrient fixation in leaf apoplasts.
EDTA vs. EDDHA Chelates
EDTA chelates provide effective micronutrient delivery in neutral to slightly acidic soils but tend to lose efficacy in alkaline conditions due to metal ion precipitation, whereas EDDHA chelates maintain high stability and bioavailability of iron and other micronutrients even in high pH soils, ensuring consistent nutrient uptake. The superior chelation strength and persistence of EDDHA complexes prevent micronutrient fixation, promoting enhanced plant growth and yield compared to traditional inorganic salts and EDTA-based formulations.
Synthetic vs. Natural Chelators
Synthetic chelators such as EDTA and DTPA provide high stability and controlled release of micronutrients like iron and zinc, enhancing nutrient availability in various soil conditions compared to natural chelators like humic acid and amino acids, which offer improved biodegradability and environmental compatibility but may have lower complex stability. Choosing between synthetic and natural chelators depends on factors such as soil pH, nutrient mobility, crop type, and sustainability goals, impacting the efficiency of micronutrient uptake and soil health in agrochemical applications.
Soil pH Buffering and Chelate Persistence
Chelated micronutrients demonstrate superior soil pH buffering capacity by maintaining nutrient availability across a wider pH range compared to inorganic salts, which are prone to precipitation and reduced solubility in alkaline or acidic conditions. The enhanced chelate persistence in the soil prevents rapid nutrient immobilization, ensuring prolonged micronutrient efficacy and improved plant uptake efficiency.
Iron Chelate Photodegradation
Iron chelates, particularly Fe-EDDHA and Fe-DTPA, exhibit enhanced stability under sunlight compared to inorganic iron salts, reducing photodegradation and ensuring sustained micronutrient availability for crops. Photodegradation of iron chelates depends on the ligand structure, with ortho-hydroxyquinoline and ethylenediaminetetraacetic acid ligands demonstrating higher resistance to UV-induced breakdown than simple inorganic salts.
Antagonistic Ion Interference
Chelated micronutrients exhibit higher absorption efficiency in plants compared to inorganic salts, as their stable complexes prevent antagonistic ion interference, minimizing nutrient precipitation and immobilization in soil. This enhances micronutrient bioavailability, promoting better plant growth and yield in agrochemical applications.
Bioavailability in Calcareous Soils
Chelated micronutrients demonstrate superior bioavailability in calcareous soils compared to inorganic salts due to their enhanced stability and resistance to precipitation at high pH levels. This increased bioavailability promotes more efficient nutrient uptake by plants, reducing micronutrient deficiencies commonly associated with alkaline soil conditions.
Micronutrient Salt Leaching Potential
Chelated micronutrients exhibit significantly lower leaching potential compared to inorganic salts due to their stable molecular structure, which enhances nutrient retention in the soil. This increased stability reduces micronutrient loss, improving plant uptake efficiency and minimizing environmental contamination.
Chelated micronutrients vs inorganic salts for micronutrient application Infographic
 agridif.com
agridif.com