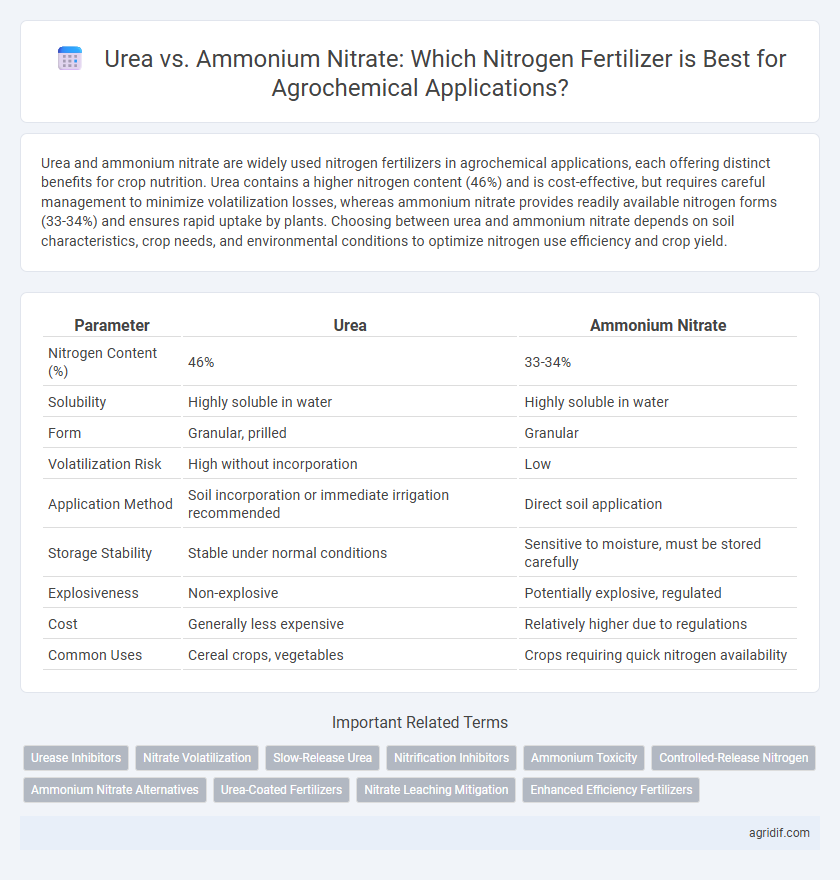
Urea and ammonium nitrate are widely used nitrogen fertilizers in agrochemical applications, each offering distinct benefits for crop nutrition. Urea contains a higher nitrogen content (46%) and is cost-effective, but requires careful management to minimize volatilization losses, whereas ammonium nitrate provides readily available nitrogen forms (33-34%) and ensures rapid uptake by plants. Choosing between urea and ammonium nitrate depends on soil characteristics, crop needs, and environmental conditions to optimize nitrogen use efficiency and crop yield.
Table of Comparison
| Parameter | Urea | Ammonium Nitrate |
|---|---|---|
| Nitrogen Content (%) | 46% | 33-34% |
| Solubility | Highly soluble in water | Highly soluble in water |
| Form | Granular, prilled | Granular |
| Volatilization Risk | High without incorporation | Low |
| Application Method | Soil incorporation or immediate irrigation recommended | Direct soil application |
| Storage Stability | Stable under normal conditions | Sensitive to moisture, must be stored carefully |
| Explosiveness | Non-explosive | Potentially explosive, regulated |
| Cost | Generally less expensive | Relatively higher due to regulations |
| Common Uses | Cereal crops, vegetables | Crops requiring quick nitrogen availability |
Understanding Nitrogen Fertilizers: Urea and Ammonium Nitrate
Urea and ammonium nitrate are two of the most widely used nitrogen fertilizers, each with distinct chemical properties affecting their efficiency and environmental impact. Urea contains 46% nitrogen by weight and requires enzymatic hydrolysis to convert into plant-available forms, while ammonium nitrate provides 33-34% nitrogen in both ammonium and nitrate forms, making it immediately accessible to crops. The choice between urea and ammonium nitrate depends on factors such as soil pH, crop type, and regional regulations, with urea favored for lower volatility when incorporated into soil and ammonium nitrate favored for rapid nitrogen availability.
Chemical Composition and Properties Comparison
Urea contains 46% nitrogen by weight, making it the highest nitrogen content solid fertilizer, while ammonium nitrate typically contains about 33-34% nitrogen, split equally between ammonium and nitrate forms. Urea's chemical formula is CO(NH2)2, which requires enzymatic hydrolysis by urease to convert into plant-available ammonium ions, whereas ammonium nitrate (NH4NO3) provides immediate availability of both ammonium and nitrate nitrogen. Urea is less hygroscopic and more stable for storage compared to ammonium nitrate, which is highly soluble and more prone to caking and explosive risks under improper handling conditions.
Mechanisms of Nitrogen Release in Soil
Urea releases nitrogen through hydrolysis, where soil urease enzymes convert urea into ammonium, which is then transformed into nitrate by nitrifying bacteria, providing a slow-release nitrogen supply. Ammonium nitrate supplies both ammonium and nitrate forms directly, allowing for immediate nitrogen availability while nitrification gradually converts ammonium to nitrate, enhancing plant uptake efficiency. Soil pH, temperature, and microbial activity significantly influence the nitrogen release rates and transformation of both urea and ammonium nitrate in the soil.
Efficiency of Nitrogen Uptake in Different Crops
Urea and ammonium nitrate serve as primary nitrogen sources but differ in nitrogen uptake efficiency across crops. Urea offers high nitrogen content (46%) and favorable soil mobility, promoting rapid absorption in cereals like wheat and rice. Ammonium nitrate, with balanced ammonium and nitrate forms, enhances nitrogen availability for crops like maize and sugarcane, improving uptake efficiency and minimizing nitrogen losses.
Impact on Soil pH and Microbial Activity
Urea releases ammonium which temporarily raises soil pH, promoting microbial activity by enhancing enzyme function and nutrient mineralization. Ammonium nitrate acidifies the soil due to nitrification, potentially inhibiting beneficial microbial populations and reducing enzyme efficiency over time. Choosing between urea and ammonium nitrate significantly influences soil fertility by altering pH balance and microbial ecosystem dynamics.
Application Methods: Best Practices for Each Fertilizer
Urea application requires incorporation into the soil or immediate irrigation to minimize nitrogen loss through volatilization, making banding and side-dressing effective methods for maximizing nitrogen use efficiency. Ammonium nitrate, being less prone to volatilization, is best applied through broadcasting or top-dressing on the soil surface, followed by light irrigation to facilitate nutrient absorption. Selecting the appropriate application method based on soil conditions and crop type enhances nitrogen availability and reduces environmental impact in nitrogen fertilization practices.
Environmental Implications: Leaching and Volatilization Risks
Urea tends to have higher ammonia volatilization losses compared to ammonium nitrate, especially when surface-applied without incorporation, leading to significant nitrogen loss and air pollution risks. Ammonium nitrate exhibits lower volatilization but is more prone to nitrate leaching, particularly in sandy soils and areas with high rainfall, increasing groundwater contamination concerns. Managing application methods and timing is critical to minimize environmental impacts associated with both fertilizers in nitrogen fertilization practices.
Cost Analysis and Availability in Agriculture Markets
Urea is generally more cost-effective than ammonium nitrate due to its higher nitrogen content (46%) and widespread availability, making it a preferred choice for large-scale agricultural use. Ammonium nitrate, while providing a balanced nitrogen release, often comes at a higher price and faces stricter regulatory controls limiting its availability in some markets. Cost analysis highlights urea's affordability and supply stability as key factors driving its dominance in nitrogen fertilization across diverse crop systems.
Safety Considerations and Handling Regulations
Urea presents lower explosion risks compared to ammonium nitrate, making it safer for storage and transport under typical agricultural conditions. Ammonium nitrate requires strict compliance with regulatory guidelines due to its high oxidizing potential and susceptibility to detonation under confinement or contamination. Proper handling protocols, including segregation from combustible materials and moisture control, are critical to prevent incidents involving ammonium nitrate in nitrogen fertilization practices.
Choosing the Right Fertilizer: Key Factors for Farmers
Urea offers the highest nitrogen content at 46%, making it cost-effective for broad applications, but its susceptibility to volatilization requires careful management. Ammonium nitrate provides a balanced nitrogen release with 33-34% nitrogen, reducing the risk of nitrogen loss and supporting quicker plant uptake. Farmers should consider soil type, crop nitrogen demand, local climate, and application timing to select the most efficient and environmentally sustainable fertilizer.
Related Important Terms
Urease Inhibitors
Urease inhibitors enhance urea efficiency by slowing the hydrolysis of urea into ammonia, reducing nitrogen losses through volatilization compared to ammonium nitrate, which does not require such inhibitors. This leads to improved nitrogen retention in the soil, higher crop uptake, and increased yield potential when using urea-based fertilization with urease inhibitors.
Nitrate Volatilization
Urea releases ammonia gas through nitrate volatilization, leading to significant nitrogen loss if not properly incorporated into the soil, whereas ammonium nitrate provides a more stable nitrogen source with minimal volatilization due to its balanced ammonium and nitrate content. Effective management of urease inhibitors and soil conditions is essential to reduce volatilization and optimize nitrogen use efficiency in urea applications.
Slow-Release Urea
Slow-release urea enhances nitrogen efficiency by gradually releasing nitrogen, minimizing leaching and volatilization compared to ammonium nitrate, which provides a rapid but short-lived nitrogen supply. This controlled nutrient delivery supports sustained crop growth, reducing environmental impact and improving fertilizer use efficiency in agrochemical applications.
Nitrification Inhibitors
Nitrification inhibitors enhance nitrogen use efficiency by slowing the conversion of ammonium to nitrate, reducing leaching and nitrous oxide emissions in both urea and ammonium nitrate applications. While urea requires hydrolysis before ammonium formation, nitrification inhibitors effectively prolong ammonium availability from ammonium nitrate, optimizing nitrogen uptake and minimizing environmental impact in agrochemical fertilization practices.
Ammonium Toxicity
Ammonium nitrate provides nitrogen in both ammonium and nitrate forms, but excessive ammonium levels can cause ammonium toxicity, resulting in inhibited root growth and nutrient uptake. Urea, which is converted into ammonium gradually, typically reduces the risk of ammonium toxicity, making it a safer choice for sensitive crops and soils with low alkalinity.
Controlled-Release Nitrogen
Urea-based controlled-release nitrogen fertilizers offer higher nitrogen content and lower volatilization losses compared to ammonium nitrate, enhancing nutrient use efficiency and crop yield stability. In contrast, ammonium nitrate's rapid nitrogen availability suits short-term crop needs but presents greater leaching risks, making urea formulations preferable for sustained nitrogen release in precision agriculture.
Ammonium Nitrate Alternatives
Ammonium nitrate alternatives for nitrogen fertilization include urea, calcium ammonium nitrate (CAN), and ammonium sulfate, each offering distinct release patterns and nutrient availability suited to different soil types and crop requirements. Urea is favored for its high nitrogen content and cost-effectiveness, but alternatives like ammonium sulfate provide additional sulfur, enhancing soil fertility and plant growth.
Urea-Coated Fertilizers
Urea-coated fertilizers offer controlled nitrogen release, reducing volatilization losses compared to ammonium nitrate, thereby enhancing nitrogen use efficiency and crop yield. Their improved solubility and slow-release properties make them a preferred choice for sustainable nitrogen fertilization in agrochemical applications.
Nitrate Leaching Mitigation
Urea and ammonium nitrate serve as common nitrogen fertilizers, but urea's slower conversion to nitrate reduces nitrate leaching risks compared to ammonium nitrate, which releases readily leachable nitrate ions. Implementing urease inhibitors with urea further mitigates nitrate leaching by slowing nitrogen transformation and enhancing nitrogen use efficiency in agrochemical applications.
Enhanced Efficiency Fertilizers
Urea and ammonium nitrate differ significantly in nitrogen release rates, with enhanced efficiency fertilizers (EEFs) like urease and nitrification inhibitors improving urea's nitrogen use efficiency by reducing volatilization and leaching. Ammonium nitrate, while providing quicker nitrogen availability, often lacks the controlled-release benefits of EEF-treated urea, making urea with EEFs a preferred option for sustaining crop nitrogen uptake and minimizing environmental impact.
Urea vs Ammonium nitrate for nitrogen fertilization Infographic
 agridif.com
agridif.com