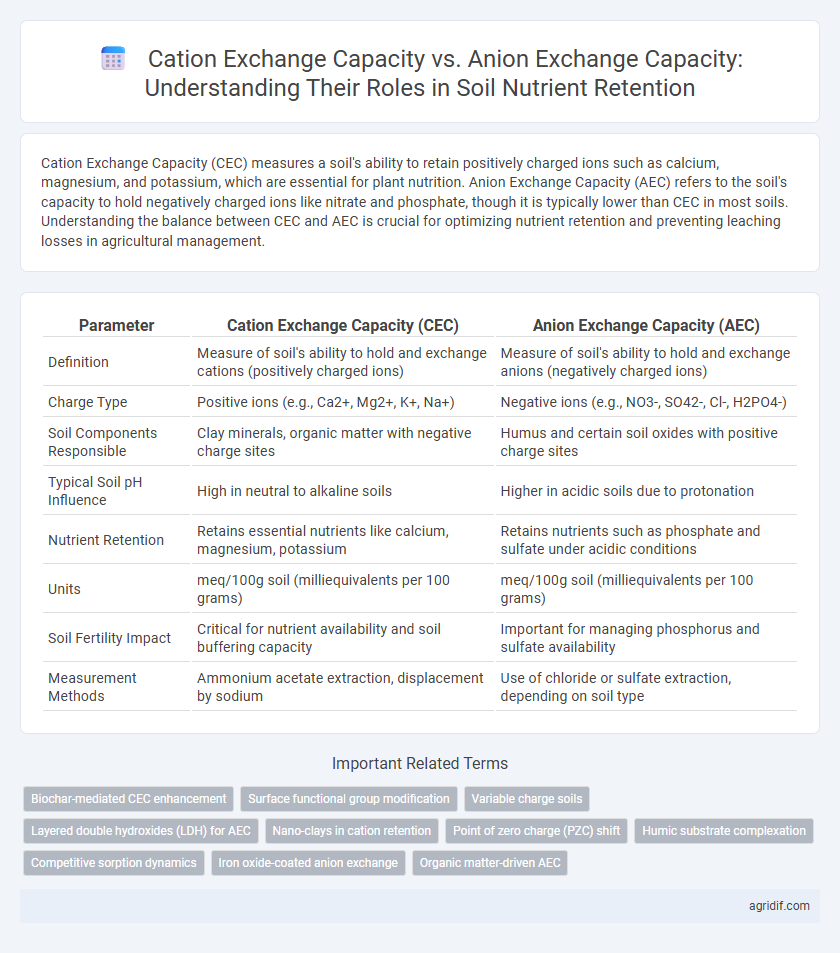
Cation Exchange Capacity (CEC) measures a soil's ability to retain positively charged ions such as calcium, magnesium, and potassium, which are essential for plant nutrition. Anion Exchange Capacity (AEC) refers to the soil's capacity to hold negatively charged ions like nitrate and phosphate, though it is typically lower than CEC in most soils. Understanding the balance between CEC and AEC is crucial for optimizing nutrient retention and preventing leaching losses in agricultural management.
Table of Comparison
| Parameter | Cation Exchange Capacity (CEC) | Anion Exchange Capacity (AEC) |
|---|---|---|
| Definition | Measure of soil's ability to hold and exchange cations (positively charged ions) | Measure of soil's ability to hold and exchange anions (negatively charged ions) |
| Charge Type | Positive ions (e.g., Ca2+, Mg2+, K+, Na+) | Negative ions (e.g., NO3-, SO42-, Cl-, H2PO4-) |
| Soil Components Responsible | Clay minerals, organic matter with negative charge sites | Humus and certain soil oxides with positive charge sites |
| Typical Soil pH Influence | High in neutral to alkaline soils | Higher in acidic soils due to protonation |
| Nutrient Retention | Retains essential nutrients like calcium, magnesium, potassium | Retains nutrients such as phosphate and sulfate under acidic conditions |
| Units | meq/100g soil (milliequivalents per 100 grams) | meq/100g soil (milliequivalents per 100 grams) |
| Soil Fertility Impact | Critical for nutrient availability and soil buffering capacity | Important for managing phosphorus and sulfate availability |
| Measurement Methods | Ammonium acetate extraction, displacement by sodium | Use of chloride or sulfate extraction, depending on soil type |
Introduction to Soil Exchange Capacities
Cation Exchange Capacity (CEC) measures a soil's ability to hold and exchange positively charged ions, crucial for nutrient retention and availability to plants. Anion Exchange Capacity (AEC) refers to the soil's capacity to adsorb negatively charged ions but is generally lower in most soils compared to CEC, impacting nutrient dynamics like phosphate and nitrate availability. Understanding the balance between CEC and AEC is essential for effective soil fertility management and optimizing nutrient uptake.
Defining Cation Exchange Capacity (CEC)
Cation Exchange Capacity (CEC) measures a soil's ability to hold and exchange positively charged ions such as calcium, magnesium, potassium, and ammonium, which are essential for nutrient availability and plant growth. CEC is influenced by soil texture, organic matter content, and pH, with higher CEC indicating greater fertility and nutrient retention capacity. Unlike Anion Exchange Capacity (AEC), which deals with negatively charged ions, CEC primarily determines the soil's capacity to supply vital macronutrients to plants.
Understanding Anion Exchange Capacity (AEC)
Anion Exchange Capacity (AEC) measures a soil's ability to retain and supply essential negatively charged nutrients such as nitrate, phosphate, and sulfate ions, playing a critical role in nutrient availability and preventing leaching losses. Unlike Cation Exchange Capacity (CEC), which focuses on positively charged ions, AEC reflects the soil's capacity to hold anions, particularly in soils with variable charge minerals like those rich in organic matter and iron or aluminum oxides. Understanding AEC is crucial for managing nutrient dynamics in acidic soils where anion retention influences fertilization efficiency and overall soil fertility.
Key Differences: CEC vs. AEC
Cation Exchange Capacity (CEC) measures a soil's ability to hold and exchange positively charged ions such as calcium, magnesium, and potassium, which are crucial for plant nutrition and soil fertility. Anion Exchange Capacity (AEC) reflects the soil's capacity to retain negatively charged ions like nitrate, phosphate, and sulfate, influencing nutrient availability and leaching potential. The key difference lies in the charge type and nutrient interactions, with CEC predominantly affecting basic cations and AEC controlling acidic anions, both essential for balanced nutrient management in soils.
Mechanisms of Nutrient Holding in Soils
Cation Exchange Capacity (CEC) measures a soil's ability to hold and exchange positively charged ions like potassium, calcium, and magnesium through negatively charged clay and organic matter surfaces, essential for nutrient retention and availability. Anion Exchange Capacity (AEC) refers to the soil's capacity to retain negatively charged ions such as nitrate and phosphate, which is influenced by variable charges on mineral surfaces, often in highly weathered or acidic soils. These mechanisms are critical for nutrient holding as CEC predominates in most soils due to abundant negative charges, while AEC plays a significant role in specific soil types, affecting overall nutrient dynamics and soil fertility management.
Impact of Soil pH on CEC and AEC
Cation Exchange Capacity (CEC) typically increases with rising soil pH due to enhanced negative charge sites on clay minerals and organic matter, facilitating greater nutrient retention of essential cations like calcium, magnesium, and potassium. Conversely, Anion Exchange Capacity (AEC) is more prominent in acidic soils where lower pH promotes positive charge sites, enhancing the soil's ability to hold anions such as phosphate and sulfate. Understanding the dynamic relationship between soil pH and exchange capacities is crucial for optimizing nutrient availability and improving soil fertility management strategies.
Role of Soil Texture and Organic Matter
Soil texture significantly influences Cation Exchange Capacity (CEC) as clay particles possess high surface area and negative charge sites that enhance nutrient retention, while sandy soils exhibit lower CEC due to larger particle size and reduced charge sites. Organic matter contributes extensively to both CEC and Anion Exchange Capacity (AEC) by providing numerous charged functional groups, improving the soil's ability to retain essential nutrients like calcium, potassium, sulfate, and nitrate. Soils rich in organic matter and fine clay fractions demonstrate superior nutrient holding capacity, supporting plant growth through balanced cation and anion exchange processes.
Practical Implications for Fertilizer Management
Cation Exchange Capacity (CEC) primarily influences soil's ability to retain essential nutrient cations such as potassium, calcium, and magnesium, directly impacting fertilizer nutrient availability and reducing leaching. In contrast, Anion Exchange Capacity (AEC) governs the soil's capacity to hold anions like nitrate and phosphate, affecting the efficiency of nitrogen and phosphorus fertilizers in acidic soils. Understanding the balance between CEC and AEC allows for targeted fertilizer management strategies, optimizing nutrient retention and minimizing environmental losses in diverse soil types.
Enhancing Soil Exchange Capacities for Crop Productivity
Cation Exchange Capacity (CEC) and Anion Exchange Capacity (AEC) are critical soil properties influencing nutrient retention and availability for crop growth. Enhancing soil CEC improves the soil's ability to retain essential cations like calcium, magnesium, and potassium, while increasing AEC facilitates the retention of vital anions such as nitrate and phosphate, directly boosting nutrient efficiency. Optimizing both exchange capacities through organic matter amendments and clay mineral management increases soil fertility, promotes nutrient balance, and enhances overall crop productivity.
Conclusion: Optimizing Soil Nutrient Retention
Cation Exchange Capacity (CEC) plays a crucial role in nutrient retention by enabling soils to hold essential positively charged nutrients such as potassium, calcium, and magnesium, directly influencing soil fertility. Anion Exchange Capacity (AEC), while typically lower in most soils, is vital for retaining negatively charged nutrients like nitrate and phosphate, preventing their leaching and enhancing nutrient availability. Optimizing both CEC and AEC through soil amendments and organic matter incorporation significantly improves nutrient retention efficiency, promoting sustainable soil fertility management.
Related Important Terms
Biochar-mediated CEC enhancement
Biochar significantly enhances soil Cation Exchange Capacity (CEC) by increasing negative charge sites, promoting nutrient retention and availability for plants, while Anion Exchange Capacity (AEC) remains comparatively low due to biochar's inherent surface chemistry. This selective CEC improvement facilitated by biochar amendment optimizes soil fertility by effectively holding essential cations like potassium, calcium, and magnesium, crucial for plant nutrition in acidic and nutrient-depleted soils.
Surface functional group modification
Surface functional group modification enhances soil's Cation Exchange Capacity (CEC) by increasing negatively charged sites such as carboxyl and hydroxyl groups, which attract and retain essential cations like calcium, magnesium, and potassium. In contrast, Anion Exchange Capacity (AEC) is influenced by positively charged functional groups, often arising under acidic conditions, facilitating the retention of anions such as phosphate and nitrate critical for nutrient availability.
Variable charge soils
Variable charge soils exhibit higher cation exchange capacity (CEC) under alkaline conditions due to increased negative surface charges, enhancing nutrient retention of essential cations like calcium, magnesium, and potassium. In contrast, anion exchange capacity (AEC) becomes more prominent in acidic environments where positive surface charges increase, facilitating the retention of vital anions such as phosphate and sulfate essential for plant nutrition.
Layered double hydroxides (LDH) for AEC
Cation Exchange Capacity (CEC) predominantly measures a soil's ability to retain essential cations like Ca2+, Mg2+, and K+, crucial for nutrient availability and soil fertility, whereas Anion Exchange Capacity (AEC), particularly in Layered Double Hydroxides (LDHs), enhances the soil's capacity to adsorb anions such as nitrate (NO3-) and phosphate (PO43-), improving nutrient retention and reducing leaching. LDHs exhibit unique positively charged layers that enable high AEC, making them critical in soils with low natural AEC for sustaining anion nutrient availability.
Nano-clays in cation retention
Nano-clays exhibit a high cation exchange capacity (CEC) due to their large surface area and negatively charged sites, enabling efficient retention of essential nutrient cations like calcium, magnesium, and potassium. In contrast, anion exchange capacity (AEC) in soils is generally lower, as fewer positively charged sites exist on nano-clay surfaces, limiting their ability to retain nutrient anions such as nitrate and phosphate.
Point of zero charge (PZC) shift
Cation Exchange Capacity (CEC) and Anion Exchange Capacity (AEC) determine soil nutrient retention, with CEC typically higher in acidic soils and AEC more prominent in alkaline conditions. The Point of Zero Charge (PZC) shift influences nutrient availability by altering soil particle charge properties, thus affecting the balance between cation and anion exchange sites critical for plant nutrient uptake.
Humic substrate complexation
Cation Exchange Capacity (CEC) in soils is predominantly influenced by the humic substrate complexation, allowing negatively charged humic substances to retain essential nutrient cations like calcium, magnesium, and potassium effectively. In contrast, Anion Exchange Capacity (AEC) in humic soils is generally lower but plays a critical role in holding anions such as nitrate and phosphate, thereby complementing nutrient availability within the soil matrix.
Competitive sorption dynamics
Cation Exchange Capacity (CEC) reflects a soil's ability to retain positively charged ions like potassium, calcium, and magnesium, playing a critical role in nutrient availability and retention. In contrast, Anion Exchange Capacity (AEC) governs the adsorption of negatively charged nutrients such as nitrate and phosphate, with competitive sorption dynamics influenced by soil pH, clay minerals, and organic matter content determining the balance between nutrient retention and leaching.
Iron oxide-coated anion exchange
Iron oxide-coated soils exhibit high anion exchange capacity (AEC) crucial for retaining negatively charged nutrients like phosphate, contrasting with cation exchange capacity (CEC) which primarily holds positively charged nutrients such as potassium and calcium. This AEC enhancement in iron oxide coatings significantly improves nutrient availability and retention in acidic soils, influencing soil fertility management and crop productivity.
Organic matter-driven AEC
Cation Exchange Capacity (CEC) primarily influences nutrient retention by holding essential cations like calcium, magnesium, and potassium, whereas Anion Exchange Capacity (AEC) driven by organic matter effectively retains vital anions such as phosphate and nitrate in acidic soils. Organic matter significantly enhances AEC due to its functional groups, improving nutrient availability and reducing leaching of anions compared to mineral-dominated soil components.
Cation Exchange Capacity vs Anion Exchange Capacity for nutrient holding Infographic
 agridif.com
agridif.com