Soil acidity, characterized by a pH below 7, often limits nutrient availability and can lead to toxic levels of aluminum and manganese, negatively affecting crop growth. Soil alkalinity, with a pH above 7, reduces the availability of essential nutrients like iron, phosphorus, and zinc, which can cause deficiencies and poor crop yields. Understanding the balance between soil acidity and alkalinity is crucial for selecting suitable crops and managing soil amendments to optimize productivity.
Table of Comparison
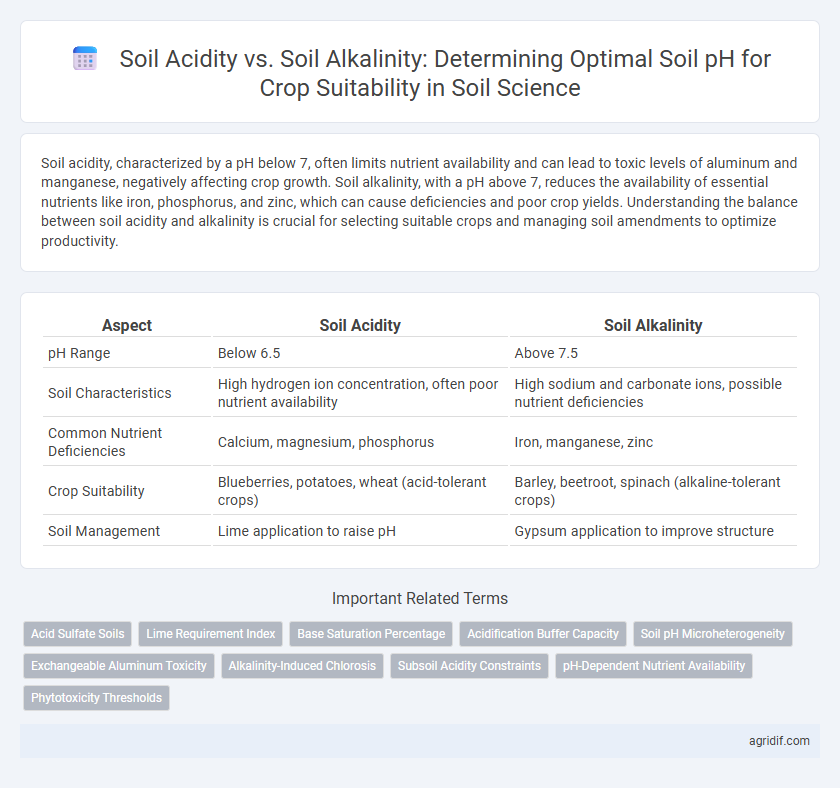
| Aspect | Soil Acidity | Soil Alkalinity |
|---|---|---|
| pH Range | Below 6.5 | Above 7.5 |
| Soil Characteristics | High hydrogen ion concentration, often poor nutrient availability | High sodium and carbonate ions, possible nutrient deficiencies |
| Common Nutrient Deficiencies | Calcium, magnesium, phosphorus | Iron, manganese, zinc |
| Crop Suitability | Blueberries, potatoes, wheat (acid-tolerant crops) | Barley, beetroot, spinach (alkaline-tolerant crops) |
| Soil Management | Lime application to raise pH | Gypsum application to improve structure |
Understanding Soil Acidity and Alkalinity
Soil acidity and alkalinity, measured by pH levels, significantly influence nutrient availability and crop suitability, with optimal growth typically occurring in soils ranging from pH 6 to 7. Acidic soils (pH below 6) often limit the availability of essential nutrients like phosphorus, calcium, and magnesium, while promoting toxic levels of aluminum and manganese, negatively impacting root development and crop yield. Alkaline soils (pH above 7) can cause deficiencies in micronutrients such as iron, zinc, and copper, making site-specific amendments crucial for crop productivity and soil health.
Causes of Acidic and Alkaline Soils
Soil acidity primarily results from high rainfall causing leaching of basic cations such as calcium, magnesium, potassium, and sodium, leaving behind hydrogen and aluminum ions that lower soil pH. Alkaline soils often develop in arid regions where limited rainfall leads to the accumulation of sodium carbonate and bicarbonate, raising soil pH. Both acidic and alkaline conditions influence nutrient availability and microbial activity, critically affecting crop suitability and productivity.
Measuring Soil pH Levels Accurately
Accurately measuring soil pH levels is essential for determining crop suitability, as soil acidity or alkalinity directly affects nutrient availability and microbial activity. Using calibrated pH meters or reliable soil test kits ensures precise readings, enabling effective soil amendments such as lime application for acidic soils or sulfur for alkaline conditions. Consistent monitoring of soil pH supports optimized crop growth by maintaining the ideal pH range preferred by specific plants.
Impact of Soil pH on Nutrient Availability
Soil pH critically influences nutrient availability by affecting the chemical forms of essential elements, with acidic soils (pH below 6) often limiting the availability of phosphorus, calcium, and magnesium while increasing the solubility of toxic metals like aluminum and manganese. In alkaline soils (pH above 7.5), micronutrients such as iron, zinc, and manganese become less soluble, leading to deficiencies that can impair crop growth and yield. Maintaining optimal soil pH, typically between 6 and 7 for most crops, ensures balanced nutrient availability and promotes effective nutrient uptake essential for healthy plant development.
Crop Suitability for Acidic Soils
Certain crops, such as blueberries, potatoes, and azaleas, thrive in acidic soils with a pH below 6.0 due to their tolerance to high aluminum availability and nutrient solubility at low pH levels. Acidic soils often require careful management of soil nutrients, particularly phosphorus and calcium, to optimize plant growth and crop yield. Selecting acid-tolerant crop varieties and utilizing soil amendments like lime can improve crop suitability and productivity in acidic soil conditions.
Crop Suitability for Alkaline Soils
Alkaline soils, characterized by a pH above 7.5, often contain high levels of sodium carbonate, which can impede nutrient availability and restrict crop growth. Crops such as barley, cotton, sugar beet, and barley thrive in these conditions due to their tolerance to high pH and sodium levels. Managing soil alkalinity through gypsum application and organic matter incorporation enhances crop suitability by improving soil structure and nutrient uptake.
Managing Soil Acidity for Optimal Yields
Managing soil acidity is critical for optimizing crop yields, as acidic soils often limit nutrient availability and inhibit root growth. Applying lime or other alkaline amendments effectively raises soil pH, enhancing microbial activity and nutrient uptake essential for crops like maize and soybeans. Regular soil testing and targeted amendments ensure balanced pH levels, promoting healthier crops and improved agricultural productivity.
Approaches to Ameliorate Alkaline Soils
Approaches to ameliorate alkaline soils involve applying organic matter such as compost or manure to increase soil microbial activity and enhance nutrient availability. The use of acidifying agents like elemental sulfur or gypsum helps lower soil pH and displace sodium ions, improving soil structure and crop suitability. Proper irrigation management and selecting salt-tolerant crop varieties also mitigate the detrimental effects of soil alkalinity on plant growth.
Case Studies: Crop Response to Soil pH Variations
Case studies reveal that crops like blueberries and potatoes exhibit optimal growth in acidic soils with pH levels between 4.5 and 5.5, while crops such as cabbage and spinach thrive in slightly alkaline soils with pH ranging from 7.0 to 7.5. Soil pH directly influences nutrient availability; for example, micronutrients like iron and manganese become less available in alkaline conditions, leading to deficiencies that affect crop yield. Tailoring soil pH management strategies based on specific crop tolerance enhances productivity and ensures sustainable agricultural practices.
Best Practices for Soil pH Management in Agriculture
Managing soil pH effectively involves regular testing to determine acidity or alkalinity, ensuring optimal crop suitability by maintaining pH levels typically between 6.0 and 7.5 for most crops. Applying lime to acidic soils raises pH and enhances nutrient availability, while sulfur or acidifying fertilizers lower alkaline soil pH to improve crop growth. Integrating organic matter improves buffer capacity, stabilizes pH fluctuations, and promotes beneficial microbial activity essential for nutrient cycling in agricultural soils.
Related Important Terms
Acid Sulfate Soils
Acid sulfate soils, characterized by their low pH values typically below 4.5, pose significant challenges for crop suitability due to the presence of toxic aluminum and iron ions that inhibit root growth and nutrient uptake. Managing these acidic conditions often requires liming or the use of acid-tolerant crop varieties to enhance soil fertility and ensure sustainable agricultural productivity.
Lime Requirement Index
Soil acidity, indicated by a low pH below 6.0, often restricts nutrient availability and crop growth, necessitating lime application based on the Lime Requirement Index to neutralize soil acidity and optimize crop yield. In contrast, alkaline soils with pH above 7.5 may require different management but generally do not benefit from lime, as excess alkalinity can limit micronutrient availability and affect crop suitability.
Base Saturation Percentage
Base saturation percentage critically influences crop suitability by indicating the proportion of soil bases such as calcium, magnesium, potassium, and sodium relative to the soil's cation exchange capacity, directly affecting soil pH from acidic to alkaline conditions. Optimal base saturation levels, typically between 60% and 80%, promote nutrient availability and microbial activity essential for crop growth, while low base saturation signals acidic soil requiring lime application to enhance fertility.
Acidification Buffer Capacity
Soil acidity and alkalinity critically influence crop suitability, with Acidification Buffer Capacity (ABC) determining a soil's resistance to pH changes and its ability to maintain optimal conditions for nutrient availability. High ABC soils, typically rich in clay and organic matter, stabilize pH levels, reducing harmful acidification effects that impair root development and nutrient uptake essential for crop growth.
Soil pH Microheterogeneity
Soil pH microheterogeneity significantly impacts crop suitability by influencing nutrient availability and microbial activity within localized soil zones, where acidic pockets may limit calcium and magnesium uptake while alkaline areas can restrict iron and manganese absorption. Understanding this fine-scale pH variability allows for targeted soil amendments, improving crop growth and yield by optimizing root zone conditions tailored to specific plant species' pH preferences.
Exchangeable Aluminum Toxicity
Soil acidity increases exchangeable aluminum toxicity, which inhibits root growth and nutrient uptake, severely limiting crop suitability, especially for aluminum-sensitive plants like beans and wheat. Soil alkalinity reduces aluminum availability, promoting better root development and nutrient absorption, thus enhancing crop yield potential in alkaline soils.
Alkalinity-Induced Chlorosis
Soil alkalinity, characterized by elevated pH levels above 7.5, often leads to alkalinity-induced chlorosis, a condition where iron becomes insoluble and unavailable to crops, causing yellowing leaves and stunted growth. Crop suitability in alkaline soils requires selecting tolerant species or applying soil amendments like sulfur or iron chelates to improve iron availability and mitigate chlorosis effects.
Subsoil Acidity Constraints
Subsoil acidity, often caused by aluminum toxicity and low pH levels below 5.5, restricts root growth and nutrient uptake, severely limiting crop suitability in acidic soils. Crop species like legumes and some cereals exhibit sensitivity, while acid-tolerant varieties or liming practices improve productivity on acidic subsoils.
pH-Dependent Nutrient Availability
Soil acidity and alkalinity critically influence nutrient availability, with acidic soils (pH below 6) limiting phosphorus, calcium, and magnesium uptake, while alkaline soils (pH above 7.5) reduce the availability of iron, manganese, and zinc essential for crop growth. Optimal crop suitability often requires maintaining soil pH between 6 and 7 to maximize nutrient solubility and ensure balanced access to macro- and micronutrients.
Phytotoxicity Thresholds
Soil acidity typically lowers pH below 5.5, increasing phytotoxicity risks from aluminum and manganese, while soil alkalinity raises pH above 7.5, causing deficiencies in iron, zinc, and phosphorus essential for crop growth. Understanding precise phytotoxicity thresholds--such as aluminum toxicity becoming critical below pH 5.0 and iron deficiency above pH 7.5--guides the selection of acid- or alkaline-tolerant crops to optimize yield and soil health.
Soil Acidity vs Soil Alkalinity for crop suitability Infographic
 agridif.com
agridif.com