Foodborne virus detection requires highly sensitive molecular techniques such as RT-PCR to identify viral RNA, while bacterial pathogen detection often relies on culture-based methods and biochemical assays. Viruses typically present lower infectious doses and less environmental stability, posing unique challenges for outbreak tracing compared to bacteria. Advances in metagenomics and biosensor technologies are enhancing the accuracy and speed of detecting both viral and bacterial contaminants in food safety investigations.
Table of Comparison
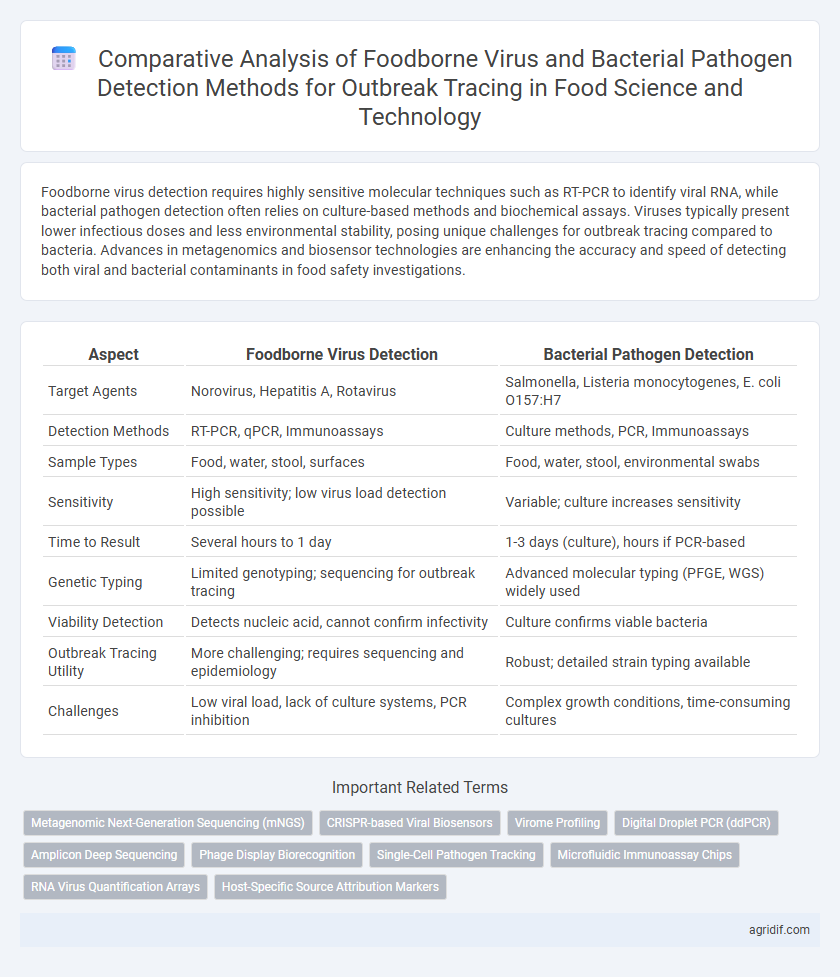
| Aspect | Foodborne Virus Detection | Bacterial Pathogen Detection |
|---|---|---|
| Target Agents | Norovirus, Hepatitis A, Rotavirus | Salmonella, Listeria monocytogenes, E. coli O157:H7 |
| Detection Methods | RT-PCR, qPCR, Immunoassays | Culture methods, PCR, Immunoassays |
| Sample Types | Food, water, stool, surfaces | Food, water, stool, environmental swabs |
| Sensitivity | High sensitivity; low virus load detection possible | Variable; culture increases sensitivity |
| Time to Result | Several hours to 1 day | 1-3 days (culture), hours if PCR-based |
| Genetic Typing | Limited genotyping; sequencing for outbreak tracing | Advanced molecular typing (PFGE, WGS) widely used |
| Viability Detection | Detects nucleic acid, cannot confirm infectivity | Culture confirms viable bacteria |
| Outbreak Tracing Utility | More challenging; requires sequencing and epidemiology | Robust; detailed strain typing available |
| Challenges | Low viral load, lack of culture systems, PCR inhibition | Complex growth conditions, time-consuming cultures |
Overview of Foodborne Viruses and Bacterial Pathogens
Foodborne viruses such as norovirus and hepatitis A are primary agents in viral outbreaks, transmitted mainly through contaminated food or water, with detection often relying on molecular techniques like RT-PCR to identify viral RNA. Bacterial pathogens including Salmonella, Listeria monocytogenes, and E. coli O157:H7 are frequently traced in outbreaks using culture-based methods combined with advanced molecular typing like whole genome sequencing for precise source attribution. Effective outbreak tracing necessitates tailored detection strategies focused on the unique biological and genomic characteristics of viruses and bacteria to improve food safety and public health interventions.
Key Differences in Detection Techniques
Foodborne virus detection primarily relies on molecular techniques such as RT-PCR and nucleic acid amplification due to the viruses' inability to grow in conventional culture systems, contrasting with bacterial pathogen detection which often utilizes culture-based methods alongside immunoassays. Viruses require high sensitivity assays because of typically low viral loads and environmental persistence, whereas bacterial detection benefits from higher microbial counts and phenotypic characterization through selective media. Advanced genomic sequencing accelerates outbreak tracing for both, but virus identification demands stringent contamination control and targeted primer design specific to viral genomes.
Sample Collection Challenges for Viruses vs Bacteria
Sample collection for foodborne virus detection presents unique challenges compared to bacterial pathogen detection due to the typically low viral loads and the uneven distribution of viruses in food matrices, requiring larger sample volumes and highly sensitive concentration methods. Viral particles often degrade rapidly outside the host, demanding stringent cold chain maintenance and rapid processing to preserve RNA integrity, whereas bacterial pathogens are generally more resilient during collection and storage. Differences in the physical and biochemical properties between viruses and bacteria necessitate tailored sampling protocols and molecular assays to accurately trace outbreaks in food safety investigations.
Sensitivity and Specificity of Detection Methods
Foodborne virus detection methods often exhibit higher sensitivity compared to bacterial pathogen detection due to the lower infectious dose and the reliance on molecular techniques such as RT-qPCR, which can identify viral RNA with precision. Specificity in viral detection is enhanced by targeting unique viral genomes, whereas bacterial pathogen detection faces challenges from genetic diversity and the presence of closely related species, sometimes resulting in cross-reactivity. Advances in next-generation sequencing and digital PCR have improved both sensitivity and specificity in tracing foodborne outbreaks, enabling more accurate identification of viral and bacterial contaminants in complex food matrices.
Molecular Tools for Outbreak Tracing
Molecular tools such as PCR and next-generation sequencing provide rapid and precise detection of foodborne viruses like norovirus and hepatitis A virus, enabling accurate outbreak tracing at the genetic level. In contrast, bacterial pathogen detection techniques often rely on culture-based methods combined with molecular assays targeting specific genes, such as those encoding toxin production or virulence factors. High-resolution genomic fingerprinting, including whole-genome sequencing, enhances differentiation between viral strains and bacterial isolates, improving epidemiological investigations and source attribution in foodborne outbreaks.
Genome Sequencing in Virus vs Bacteria Outbreaks
Genome sequencing plays a critical role in outbreak tracing by providing precise identification and differentiation of foodborne viruses and bacterial pathogens. Viral genomes, often smaller and RNA-based, require reverse transcription before sequencing, whereas bacterial genomes are larger, DNA-based, and allow more comprehensive phylogenetic analysis. The rapid evolution and high mutation rates of viruses demand specialized sequencing platforms and bioinformatics pipelines for accurate outbreak source tracking compared to bacterial pathogen detection.
Turnaround Time and Rapid Response Capabilities
Foodborne virus detection techniques, such as RT-PCR and next-generation sequencing, offer faster turnaround times compared to traditional bacterial pathogen culture methods, enabling quicker outbreak tracing. Viral assays often provide results within hours to a day, enhancing rapid response capabilities critical for mitigating spread. In contrast, bacterial pathogen detection may require 24-72 hours due to culturing steps, delaying outbreak confirmation and intervention measures.
Limitations in Current Surveillance Systems
Current surveillance systems often exhibit limitations in detecting foodborne viruses compared to bacterial pathogens due to lower viral load and difficulties in sample concentration and purification. Viral genomes present challenges for traditional culture-based methods, leading to underreporting and delayed outbreak response. Improvements in molecular techniques like RT-PCR and metagenomics are critical for enhancing sensitivity and specificity in viral pathogen detection during foodborne illness investigations.
Future Technologies for Enhanced Detection
Emerging technologies like CRISPR-based assays and digital PCR offer unprecedented sensitivity for detecting foodborne viruses, surpassing traditional bacterial pathogen detection methods. Integrating AI-powered genomic analysis accelerates outbreak tracing by distinguishing viral mutations and bacterial strains with high precision. Future advancements in biosensor design and multiplexed sequencing platforms promise real-time, on-site detection, revolutionizing food safety monitoring.
Integrated Approaches for Effective Outbreak Management
Integrated approaches for effective outbreak management rely on combining molecular techniques such as PCR and next-generation sequencing to simultaneously detect foodborne viruses and bacterial pathogens, improving traceability and response time. Multiplex assays enable the differentiation and quantification of viral agents like norovirus alongside bacterial pathogens such as Salmonella and Listeria, enhancing surveillance accuracy. Implementing these comprehensive diagnostic tools within food safety monitoring systems facilitates rapid identification of contamination sources, reducing outbreak duration and public health impact.
Related Important Terms
Metagenomic Next-Generation Sequencing (mNGS)
Metagenomic Next-Generation Sequencing (mNGS) enables simultaneous detection of diverse foodborne viruses and bacterial pathogens with high sensitivity and specificity, crucial for precise outbreak tracing in food safety. Unlike traditional culture-based bacterial pathogen detection, mNGS provides comprehensive genomic data that facilitates rapid identification of viral genomes and antimicrobial resistance genes, enhancing response to foodborne illness outbreaks.
CRISPR-based Viral Biosensors
CRISPR-based viral biosensors provide highly specific, rapid, and sensitive detection of foodborne viruses such as norovirus and hepatitis A, outperforming traditional bacterial pathogen detection methods that rely on culturing and PCR for outbreak tracing. These biosensors leverage engineered Cas proteins for real-time viral RNA recognition, enabling point-of-care diagnostics critical for controlling viral foodborne outbreaks efficiently.
Virome Profiling
Virome profiling enables precise detection of foodborne viruses by analyzing the complete viral community within contaminated samples, surpassing traditional bacterial pathogen detection methods that primarily target specific bacterial strains. Advanced metagenomic sequencing techniques enhance outbreak tracing accuracy by identifying viral genomes, improving risk assessment and management in food safety.
Digital Droplet PCR (ddPCR)
Digital Droplet PCR (ddPCR) offers higher sensitivity and absolute quantification in detecting foodborne viruses compared to bacterial pathogens, enabling precise outbreak tracing even at low viral loads. Its partitioning capability reduces inhibition effects common in complex food matrices, making ddPCR a superior tool for reliable identification of viral contaminants in food safety monitoring.
Amplicon Deep Sequencing
Amplicon Deep Sequencing enhances foodborne virus detection by enabling high-resolution identification of viral genotypes, crucial for outbreak tracing where traditional bacterial pathogen detection methods may lack sensitivity and specificity. This technology allows simultaneous analysis of multiple viral targets directly from contaminated food samples, providing comprehensive insights into virus diversity and transmission pathways that improve food safety interventions.
Phage Display Biorecognition
Phage display biorecognition offers high specificity and sensitivity in detecting foodborne viruses by presenting diverse peptide libraries that bind targeted viral epitopes, surpassing traditional bacterial pathogen detection methods reliant on culture or PCR. This technique enables rapid outbreak tracing through precise viral identification, enhancing food safety monitoring and response strategies in food science and technology.
Single-Cell Pathogen Tracking
Single-cell pathogen tracking enhances the resolution of foodborne virus detection by isolating viral particles within individual host cells, enabling precise identification and differentiation from bacterial pathogens in outbreak tracing. This method leverages advanced fluorescent tagging and microfluidic sorting to monitor infection dynamics at the cellular level, surpassing traditional bulk detection approaches in sensitivity and specificity.
Microfluidic Immunoassay Chips
Microfluidic immunoassay chips enable rapid, sensitive detection of foodborne viruses like norovirus and hepatitis A virus, surpassing traditional bacterial pathogen assays in outbreak tracing precision due to their ability to analyze low viral loads in complex food matrices. These chips integrate immunocapture and signal amplification on a miniaturized platform, facilitating high-throughput screening critical for real-time epidemiological surveillance and reducing false negatives common in conventional bacterial culture methods.
RNA Virus Quantification Arrays
RNA virus quantification arrays enable precise detection and measurement of foodborne RNA viruses, offering higher sensitivity compared to traditional bacterial pathogen detection methods used in outbreak tracing. These arrays facilitate rapid identification of viral contamination in food matrices, improving accuracy in foodborne illness source tracking and supporting timely public health interventions.
Host-Specific Source Attribution Markers
Host-specific source attribution markers differentiate viral RNA sequences from bacterial DNA signatures, enabling precise identification of contamination sources in foodborne outbreaks. Molecular techniques targeting unique viral capsid genes outperform traditional bacterial pathogen detection in tracing host origin and transmission routes.
Foodborne virus detection vs bacterial pathogen detection for outbreak tracing Infographic
 agridif.com
agridif.com