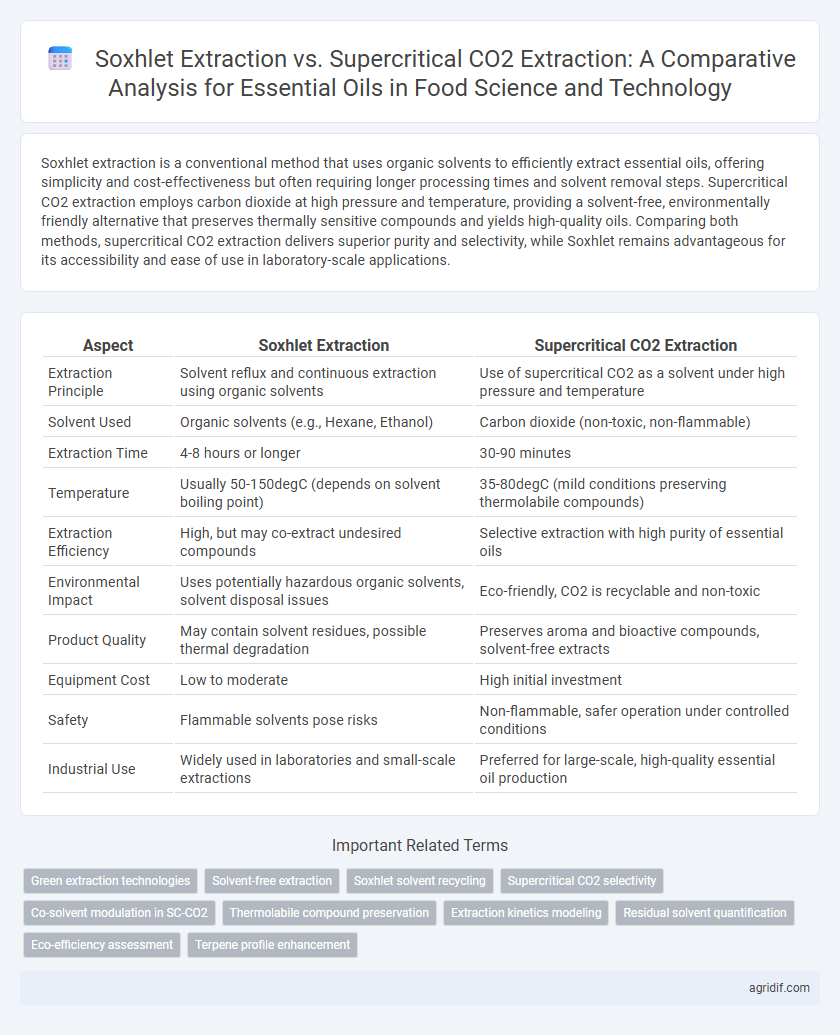
Soxhlet extraction is a conventional method that uses organic solvents to efficiently extract essential oils, offering simplicity and cost-effectiveness but often requiring longer processing times and solvent removal steps. Supercritical CO2 extraction employs carbon dioxide at high pressure and temperature, providing a solvent-free, environmentally friendly alternative that preserves thermally sensitive compounds and yields high-quality oils. Comparing both methods, supercritical CO2 extraction delivers superior purity and selectivity, while Soxhlet remains advantageous for its accessibility and ease of use in laboratory-scale applications.
Table of Comparison
| Aspect | Soxhlet Extraction | Supercritical CO2 Extraction |
|---|---|---|
| Extraction Principle | Solvent reflux and continuous extraction using organic solvents | Use of supercritical CO2 as a solvent under high pressure and temperature |
| Solvent Used | Organic solvents (e.g., Hexane, Ethanol) | Carbon dioxide (non-toxic, non-flammable) |
| Extraction Time | 4-8 hours or longer | 30-90 minutes |
| Temperature | Usually 50-150degC (depends on solvent boiling point) | 35-80degC (mild conditions preserving thermolabile compounds) |
| Extraction Efficiency | High, but may co-extract undesired compounds | Selective extraction with high purity of essential oils |
| Environmental Impact | Uses potentially hazardous organic solvents, solvent disposal issues | Eco-friendly, CO2 is recyclable and non-toxic |
| Product Quality | May contain solvent residues, possible thermal degradation | Preserves aroma and bioactive compounds, solvent-free extracts |
| Equipment Cost | Low to moderate | High initial investment |
| Safety | Flammable solvents pose risks | Non-flammable, safer operation under controlled conditions |
| Industrial Use | Widely used in laboratories and small-scale extractions | Preferred for large-scale, high-quality essential oil production |
Overview of Essential Oil Extraction Methods in Agriculture
Soxhlet extraction, a traditional solvent-based method, enables efficient extraction of essential oils by repeatedly washing plant material with a heated organic solvent, ensuring high yield but involving longer extraction times and potential solvent residues. Supercritical CO2 extraction utilizes CO2 at supercritical conditions as a non-toxic, selective solvent, offering faster extraction rates, higher purity, and preservation of thermolabile compounds critical for agricultural applications. These methods represent key approaches in agriculture to balance extraction efficiency, product quality, and environmental sustainability in essential oil production.
Principles of Soxhlet Extraction for Essential Oils
Soxhlet extraction for essential oils operates on the principle of continuous solvent reflux and percolation, where a solvent repeatedly washes the plant material to dissolve and extract the oils. This technique utilizes a closed system that allows the solvent to evaporate, condense, and drip back onto the sample, ensuring efficient extraction over time. The method is valued for its simplicity and ability to extract high yields of essential oils from solid matrices without degrading thermolabile compounds.
Fundamentals of Supercritical CO2 Extraction Technology
Supercritical CO2 extraction operates by utilizing carbon dioxide above its critical temperature (31.1degC) and pressure (73.8 bar) to achieve a solvent with gas-like diffusivity and liquid-like density, optimizing essential oil solubilization. This method enables selective extraction of thermolabile compounds without solvent residues, preserving bioactive components' integrity compared to traditional Soxhlet extraction, which relies on prolonged heating and organic solvents. The tunable solvent power of supercritical CO2, modulated through pressure and temperature adjustments, enhances extraction efficiency and environmental sustainability in essential oil recovery.
Comparative Efficiency: Soxhlet vs. Supercritical CO2 Extraction
Soxhlet extraction often yields higher quantities of essential oils but requires longer processing times and uses large volumes of organic solvents, impacting environmental safety. Supercritical CO2 extraction offers superior selectivity and preserves thermolabile compounds due to lower operating temperatures, resulting in purer essential oil extracts with minimal solvent residues. Comparative studies reveal that supercritical CO2 extraction achieves faster extraction rates and greater efficiency in recovering bioactive compounds despite higher initial equipment costs.
Solvent Use and Environmental Impact in Extraction Processes
Soxhlet extraction relies on organic solvents like hexane or ethanol, which pose significant environmental and health risks due to volatility and toxicity, leading to hazardous waste generation. Supercritical CO2 extraction uses carbon dioxide as a green solvent, offering a non-toxic, non-flammable alternative that significantly reduces solvent residues and environmental pollution. The environmentally friendly profile of supercritical CO2 extraction aligns with sustainable food science practices, minimizing the ecological footprint compared to the traditional Soxhlet method.
Quality and Purity of Essential Oils: Method-Dependent Differences
Soxhlet extraction often results in essential oils with higher solvent residues and potential thermal degradation due to prolonged heating, impacting both quality and purity. In contrast, supercritical CO2 extraction preserves the integrity of heat-sensitive compounds by operating at moderate temperatures and offers solvent-free oils with superior purity and enhanced aroma profiles. This method-dependent variation strongly influences the chemical composition and sensory characteristics of the extracted essential oils in Food Science and Technology.
Energy Consumption and Sustainability Considerations
Soxhlet extraction typically consumes higher energy due to prolonged heating cycles at elevated temperatures, whereas supercritical CO2 extraction operates under moderate temperatures and pressures with enhanced energy efficiency. Supercritical CO2 extraction supports sustainability by using non-toxic, recyclable CO2 as a solvent, minimizing environmental impact compared to organic solvents in Soxhlet extraction. The reduced solvent waste and lower carbon footprint make supercritical CO2 extraction a more sustainable choice for essential oil production in food science applications.
Application Suitability: Selecting the Right Extraction Method
Soxhlet extraction is highly effective for extracting essential oils from solid plant matrices due to its thorough solvent recycling and ability to handle high-boiling solvents, making it suitable for heat-stable compounds. Supercritical CO2 extraction offers a solvent-free, tunable process ideal for heat-sensitive essential oils, providing high purity and preserving bioactive components. Choosing the right method depends on the target compound's thermal stability, desired purity, and environmental considerations within food science and technology applications.
Advances and Innovations in Extraction Technologies
Soxhlet extraction remains a traditional method for essential oil isolation, utilizing solvent reflux cycles for efficient extraction but often requires long processing times and large volumes of organic solvents. Supercritical CO2 extraction has emerged as a cutting-edge technology, offering rapid, solvent-free extraction with enhanced selectivity and preservation of thermolabile compounds. Innovations in supercritical fluid parameters and co-solvent additives continue to improve yield, purity, and environmental sustainability in essential oil recovery.
Future Trends in Essential Oil Extraction for Food Science
Future trends in essential oil extraction for Food Science emphasize supercritical CO2 extraction due to its superior selectivity, eco-friendliness, and ability to preserve thermolabile compounds compared to Soxhlet extraction. Innovations in process optimization and scale-up for supercritical CO2 methods aim to enhance extraction efficiency, reduce solvent usage, and maintain the bioactive profile of essential oils. Emerging technologies integrating supercritical CO2 with ultrasound or microwave-assisted extraction show promise in accelerating extraction kinetics while ensuring sustainable production practices.
Related Important Terms
Green extraction technologies
Supercritical CO2 extraction offers a green alternative to Soxhlet extraction by using non-toxic CO2 and operating at lower temperatures, preserving the integrity of essential oils while reducing solvent waste. This eco-friendly method enhances extraction efficiency and selectivity, aligning with sustainable food science and technology practices.
Solvent-free extraction
Supercritical CO2 extraction offers a solvent-free, environmentally friendly method for obtaining essential oils, preserving thermolabile compounds by operating at lower temperatures compared to traditional Soxhlet extraction, which relies on organic solvents and prolonged heating. The non-toxic nature of CO2 as a solvent enhances oil purity and safety, making it preferable for food-grade essential oil production, while Soxhlet extraction may leave solvent residues and degrade sensitive components.
Soxhlet solvent recycling
Soxhlet extraction allows effective solvent recycling by continuously condensing and reusing the solvent, reducing cost and environmental impact during essential oil recovery. However, this method often involves longer extraction times and potential thermal degradation of sensitive compounds compared to supercritical CO2 extraction.
Supercritical CO2 selectivity
Supercritical CO2 extraction offers superior selectivity for essential oils by precisely tuning pressure and temperature to target specific compounds, resulting in a purer and more refined extract compared to traditional Soxhlet extraction. This method minimizes thermal degradation and solvent residues, enhancing the quality and bioactivity of the recovered essential oils.
Co-solvent modulation in SC-CO2
Supercritical CO2 extraction allows precise co-solvent modulation to enhance the solubility and selectivity of essential oils, improving yield and purity compared to traditional Soxhlet extraction, which relies on prolonged solvent exposure and higher temperatures. Co-solvent addition, such as ethanol, in SC-CO2 extraction alters polarity, enabling efficient extraction of polar compounds while preserving thermally sensitive bioactive constituents.
Thermolabile compound preservation
Soxhlet extraction exposes thermolabile compounds in essential oils to prolonged heat, risking degradation and loss of bioactivity, whereas supercritical CO2 extraction operates at lower temperatures, effectively preserving heat-sensitive constituents and maintaining the oils' phytochemical integrity. This solvent-free method also offers selective extraction parameters that enhance yield and quality of delicate volatile compounds compared to traditional solvent-based Soxhlet techniques.
Extraction kinetics modeling
Soxhlet extraction exhibits slower and less selective extraction kinetics due to solvent diffusion limitations and equilibrium constraints, whereas supercritical CO2 extraction demonstrates faster, tunable, and more efficient extraction rates driven by adjustable pressure and temperature conditions that enhance solute solubility and mass transfer. Modeling supercritical CO2 kinetics often employs diffusion and solubility-based mechanisms, providing precise control over essential oil yield and composition compared to the relatively simplistic and longer Soxhlet extraction process.
Residual solvent quantification
Soxhlet extraction often results in higher residual solvent levels, primarily due to the use of organic solvents like hexane, requiring rigorous post-extraction purification to meet safety standards. In contrast, supercritical CO2 extraction minimizes residual solvent contamination as CO2 evaporates completely under ambient conditions, ensuring purer essential oils and compliance with strict regulatory limits on solvent residues.
Eco-efficiency assessment
Soxhlet extraction, while effective for essential oil isolation, consumes significant amounts of organic solvents and energy, leading to higher environmental impacts compared to supercritical CO2 extraction, which offers solvent-free, low-temperature processing with reduced carbon footprint and improved eco-efficiency. Supercritical CO2 extraction enhances oil purity and yield while minimizing waste generation and toxicity, making it a sustainable choice in food science and technology for essential oil production.
Terpene profile enhancement
Soxhlet extraction efficiently isolates essential oils but often subjects terpenes to thermal degradation, reducing the complexity of the terpene profile. Supercritical CO2 extraction enhances terpene preservation and diversity by operating at lower temperatures and tunable pressures, resulting in a richer, more intact terpene profile in essential oils.
Soxhlet extraction vs supercritical CO2 extraction for essential oils Infographic
 agridif.com
agridif.com